Dr Carlos Ziebert, head of IAM-AWP’s Calorimeter Center, KIT, explains how battery calorimeters allow thermal characterisation and safety testing for an improved sodium-ion battery.
In late 2018, the POLiS (Post Lithium Storage) Cluster of Excellence was acquired jointly by KIT and Ulm University within the highly competitive Excellence Strategy competition of the federation and the federal states of Germany. It has a budget of about €7m per year and is scheduled for an initial duration of seven years. In this cluster, the IAM-AWP Calorimeter Center, which was established in 2011, is responsible for thermal characterisation and safety of post-lithium materials, electrodes, and cells. Post-lithium batteries use more abundant and environmentally friendly materials when compared to lithium, nickel, and cobalt. These can be for example, sodium, magnesium, or calcium.
For the first two and a half years, the focus of the cluster was on the development of a sodium-ion battery (SIB), which is currently the most mature post-lithium technology. The working principle of a sodium-ion battery is very similar to that of a lithium-ion battery (see Fig. 1). Instead of Li-ions, in a SIB Na-ions are transferred via an organic electrolyte through a separator between the two electrodes, in which they are intercalated and deintercalated respectively. The cathode is typically a transition metal oxide that can have a layered structure such as NaMnO2 or an olivine structure such as NaFePO4, which both provide defined diffusion paths for the sodium-ions.
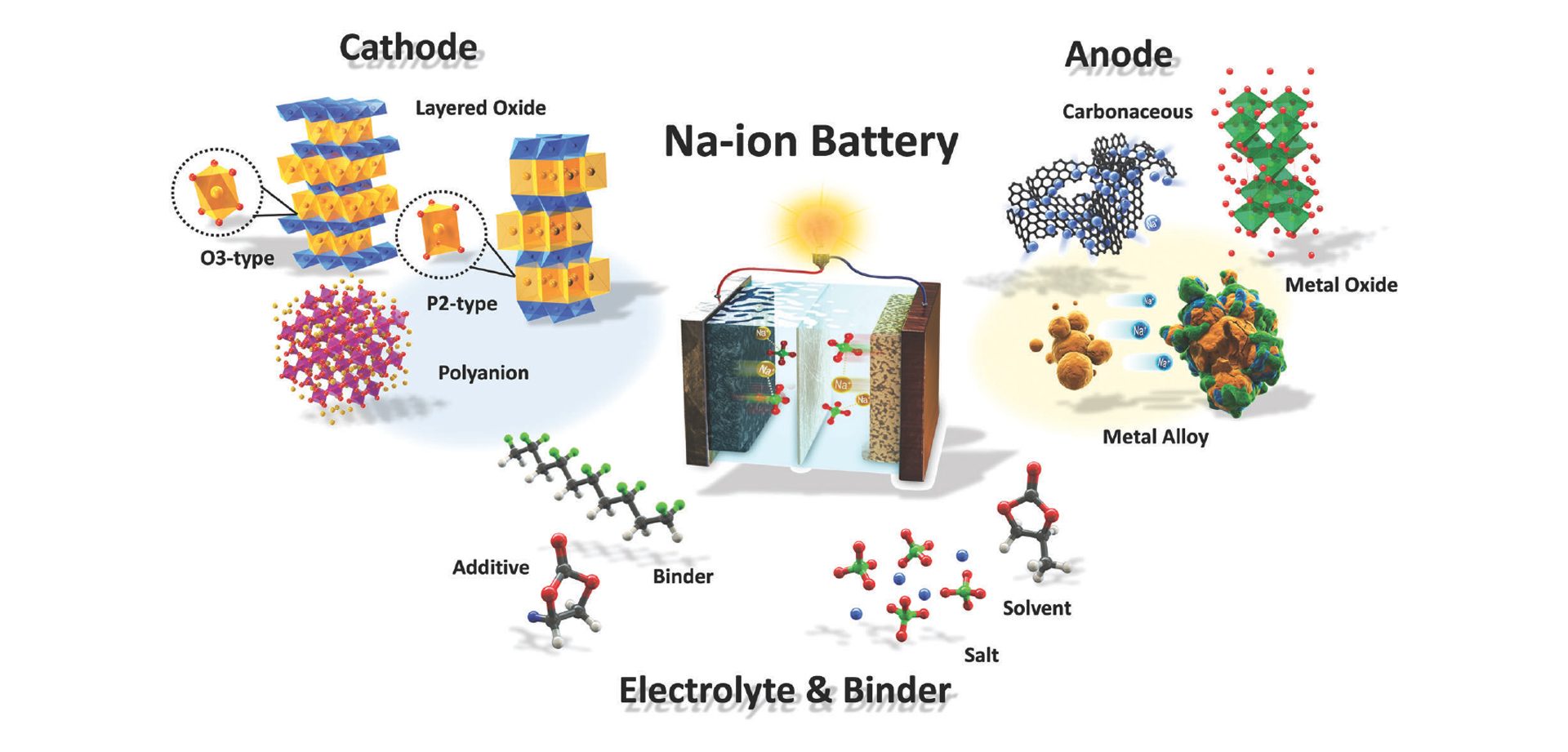
Layered cathode materials with P2-type and O3-type are particularly promising because of their high specific capacity, their broad range of working voltage, and an easy synthesis process. P2-types such as the NaMnO2 cathodes have open prismatic paths within metal oxide slabs that facilitate direct sodium-ion diffusion which might lead to better high-rate properties. However, they usually also suffer from the P2–O2 phase transformation when Na-ions are extracted, which leads to a significant volume change that causes a reduction of the reversible capacity.
On the anode side, metal alloys, metal oxides, or carbonaceous materials can be used. The most frequently proposed electrolytes are, in analogy to lithium-ion batteries, solutions of sodium salts such as sodium hexafluorophosphate or sodium perchlorate in binary or ternary mixtures of different organic carbonates such as propylene carbonate, ethylene carbonate, and diethyl carbonate with additives such as vinylene carbonate (VC) and fluorethylene carbonate (FEC).
Thermal studies
In addition to the electrochemical characterisation, the characterisation of the thermal properties is needed to achieve an in-depth understanding of the underlying reaction mechanisms and heat conduction processes, which are at the moment not well-known for most of the post-lithium cells,’ explains Dr Ijaz Ul Mohsin, the responsible scientist in Dr. Ziebert’s group Battery Calorimetry and Safety.
On the materials and components level, differential scanning calorimetry (DSC) and extremely sensible Tian-Calvet calorimeters are used to provide thermophysical parameters such as heat capacity or thermal conductivity and to analyse in detail the possible phase transformations and the thermal stability of the materials. On the small-scale cell level, larger-scale Tian-Calvet calorimeters allow the determination of the heat generation during cycling with great accuracy by direct heat flow measurement. The heat flow is determined by the 3D Tian-Calvet Sensor arrangement, where both the sample and the reference vessel are surrounded by rings with hundreds of thermocouples.
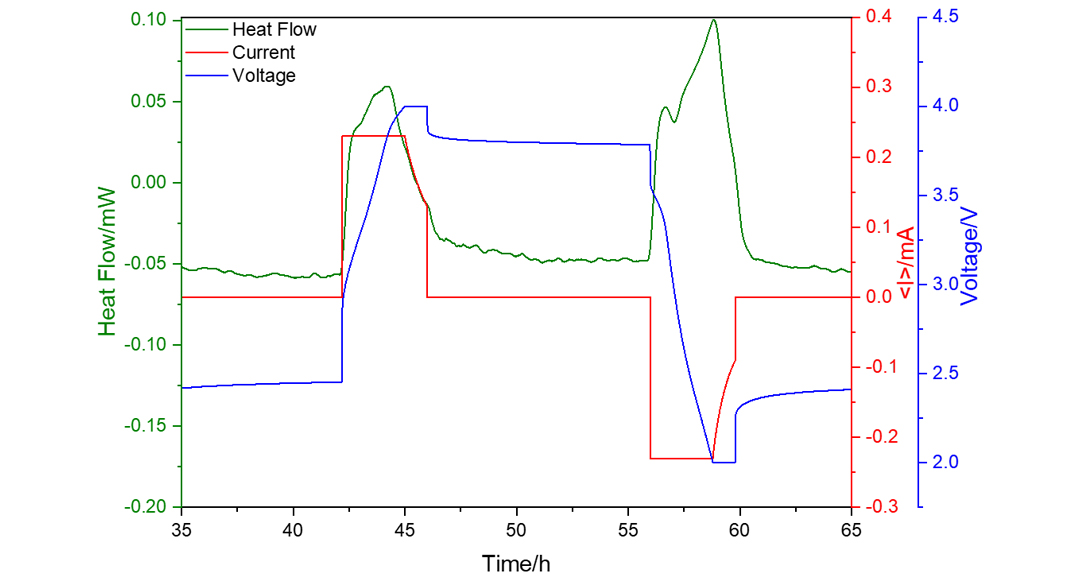
Fig. 2 shows an example of a SIB coin cell with Na0.53MnO2 cathode and hard carbon (HC) anode. Even if this cell only has a capacity of about 1 mAh, the heat flow (green curve) can be clearly measured for the charge and discharge process as indicated by the applied current (red curve) and the resulting voltage (blue curve). For a maximum current of 0.23 mA, the integration of the heat flow over time gives 1.3 J of generated heat during charging and 1.5 J during discharging. The measured heat profiles represent a fingerprint for the underlying electrochemical processes at the electrodes and are essential for SOH (state-of-health) and ageing prediction.
Safety studies
The safety of such new battery systems can only be guaranteed by starting right from the beginning, with detailed thermal and safety studies on the small-scale cell level (coin cells) in very sensitive battery calorimeters. The scale-up process then needs to be accompanied by both thermal investigations and safety tests (thermal runaway, overcharging, internal/external short circuit, mechanical impact) in battery calorimeters of larger sizes, so-called ‘Accelerating Rate Calorimeters’ (ARCs).
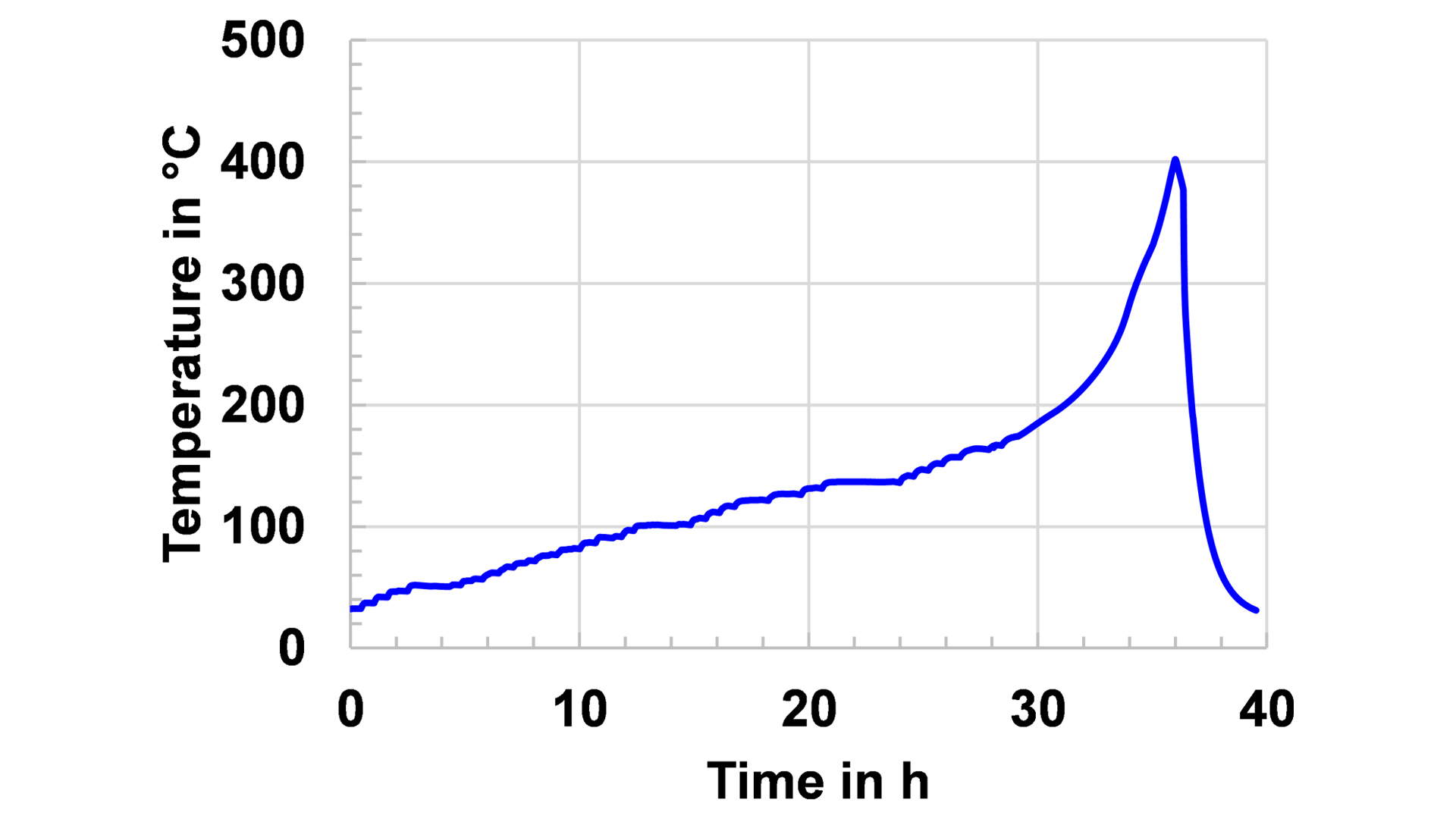
Fig. 3 illustrates a Heat-Wait-Seek (HWS) test on a stack of two identical cells as those in Fig. 2. This thermal abuse test heats the cell up in 5°C steps, and at the end of each step after a waiting period of between 15 and 45 minutes, depending on the size of the cell, the system enters into a seek phase, where it detects whether the cell generates heat that leads to a significant temperature increase. If this condition is fulfilled, the system switches to a quasi-adiabatic mode, where the heaters in the calorimeter chamber instantaneously follow the temperature change of the cells surface. This prevents the heat transfer to the chamber and consequently the cell is increasingly heated up until a thermal runaway occurs or the chemicals for this exothermal reaction are completely consumed. The plateaus in the temperature curve indicate the different stages of the reactions that end up in the thermal runaway, which leads to a maximum temperature of 400°C.
If the cell is inserted into a closed cell holder, which has an opening to a capillary connected to a pressure transducer, then a pressure increase occurs during the different reactions by the gas.
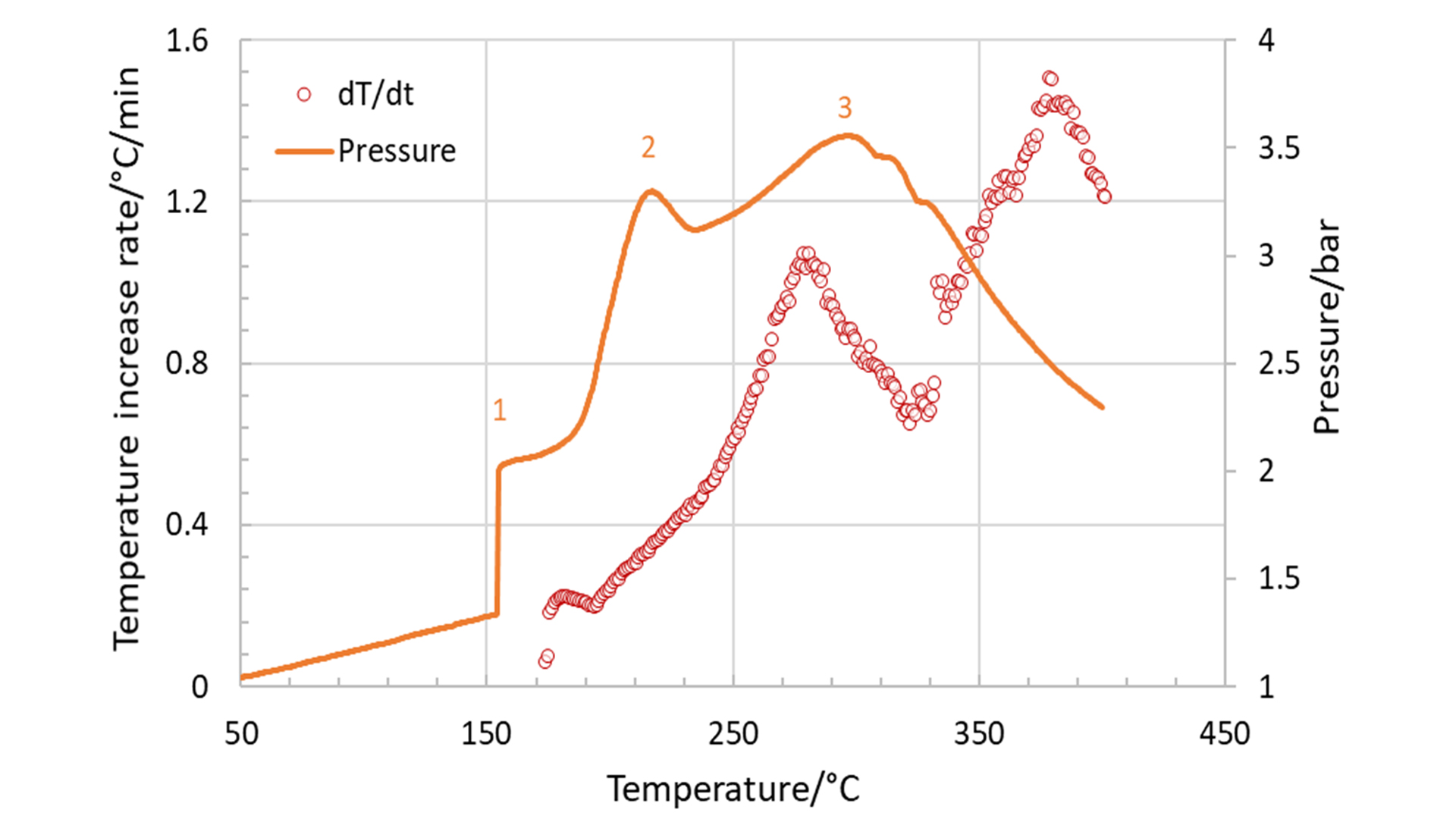
The first reaction is typically the decomposition of the solid-electrolyte interphase (SEI) layer, which is formed during the formation process of the cell and protects the anode from the electrolyte. The second stage represents the exothermic reactions between the electrolyte and the cathode, and the third stage is the decomposition of the electrode and the electrolyte that ends up in the thermal runaway. This is illustrated in Fig. 4, which shows the temperature rate (red circles) and the pressure (orange curve) both plotted against the temperature. The maximum measured values for the temperature rate and the resulting pressure were 1.5°C/min and 3.5 bar, respectively.
After the tests, post-mortem analysis of the cells is performed in order to reveal the fundamental reaction mechanisms that lead to degradation and critical failures. As a result of the investigations, quantitative and system relevant data for temperature, heat, and pressure development of materials and cells are provided as a feedback for the cell developers in order to help them to optimise the cells for the different requirements.
Calorimetry of the cells, and their individual active materials, is thus required to obtain quantitative thermal and thermodynamic data and for a holistic safety assessment. The winning post-Li technologies will win partly on their safety argument, possibly sacrificing some energy density.
To summarise: battery calorimetry is an innovative approach to provide quantitative and system relevant data for the temperature, heat, and pressure development of materials and cells as fast feedback for cell development and input data for simulations. This will help to pave the way for mature and safe sodium-ion and other post-lithium batteries.








