The CHASS project aims to improve the lifetime and efficiency of Cu-CHA catalysts for nitrogen oxides removal in freight transport.
Emission of nitrogen oxides (NOx) is a significant contributor to air pollution worldwide, and can also have detrimental consequences for both population health and social costs. The main sources of NOx emissions are power plants and combustion engines, where road transportation is an important representative. Notwithstanding the expected shift to electric vehicles, long haul transportation – an important driving force of EU economy – is still reliant on diesel engines. This means stricter legislation on air quality and greenhouse gas emissions cannot be attained without an improvement in diesel engine technology.
To reduce NOx emissions from vehicles, catalytic exhaust systems have been developed and utilised for decades. Diesel engines are widely used, and are currently the most energy efficient combustion engines. Therefore, it is highly important that improvements in diesel engine technology can reach the goals for air quality and greenhouse gas emissions.
Selective catalytic reduction can control NOx emissions
The fuel-efficiency of diesel engines is largely thanks to excess amounts of air in the combustion process, in contrast to gasoline-powered engines. Consequently, the exhaust gas of diesel engines still contains oxygen, implying that the reduction of NOx emission in diesel exhausts has to take place in the presence of oxygen.
The selective catalytic reduction (SCR) of NOx by ammonia (NH3) (NH3-SCR) is an efficient reaction that converts NOx and NH3 in the presence of oxygen to nitrogen (N2) and water (H2O), both occurring naturally in the Earth’s atmosphere. Since NH3 is not emitted from the engine, it must be added from an external source.
In the technical implementation of SCR on diesel vehicles, ammonia is added as an aqueous solution of urea (AdBlue®), which releases NH3 upon decomposition. The NH3-SCR technology for NOx reduction is applicable in all situations where oxygen is present, which is also often in the exhausts of power plants and ships.
Furthermore, the successful implementation of biofuels in diesel engines depends on controlling the emission of NOx. In all these applications, NH3-SCR is an important technology to mitigate unwanted NOx emissions.
The potential of Cu-CHA catalysts
The common catalysts for NH3-SCR on vehicles are based on vanadium oxide supported on titania, Fe-zeolites, or Cu-zeolites. Zeolites are microporous crystalline aluminosilicate materials with a typical diameter of 0.3–0.8 nanometres, commonly used as commercial adsorbents and heterogeneous catalysts. The ordered porous three-dimensional framework is formed by linking of Al and Si atoms by bridging oxygen atoms. The negative charge induced on the framework by the presence of Al atoms requires a positive counterions, which usually infer to the solid its catalytic properties.
Cu-exchanged zeolites, and Cu-CHA in particular (a small pore zeolite with the chabazite structure), performs excellently in the low temperature range (150-300°C). The focus for the development of engines is currently on improved fuel efficiency, requiring temperature in diesel exhausts systems to be lower. Consequently, the performance of SCR catalysts in the temperature range 150-300°C will be an important parameter for application in future diesel engines.
Cu-CHA-type catalysts perform well up to 550°C, and can tolerate temperatures up to 800°C. Therefore, Cu-CHA catalysts are currently the material of choice for NH3-SCR applications particularly on freight transport.
Deactivation risks of Cu-CHA catalysts and mitigation
Despite their superior stability, repeated exposure to high temperatures and the harsh environment of exhaust systems can still deactivate Cu-zeolite based catalysts – i.e. the performance deteriorates with time.
In particular, the presence of water vapour in the exhaust gas at high temperatures can damage the zeolite structure by removing Al atoms from the framework (dealumination), in a process so-called hydrothermal ageing.
Importantly, small amounts of SO2, a common component in diesel exhaust gas, can result in a severe deactivation of Cu-zeolites, most notably between 150 and 300°C, which is the range where Cu-zeolites otherwise show their superior performance.
As deactivation may result in the malfunction of the exhaust system, the applicability of Cu-CHA catalysts is limited to systems where poisoning by SO2 is less probable. In practice, this means that ultra-low sulphur diesel is required and even then, exhaust systems must be designed to handle possible deactivation by SO2.
The CHASS project and expert collaboration
The CHASS project intends to address these challenges, to understand the deactivation processes and to develop models to describe the behaviour of durable Cu-zeolite based NH3-SCR catalysts.
The project brings together leading European groups in the field of automotive catalysts with complementary expertise. The Physical Chemistry experimental group at the University of Torino in Italy, the Computational Catalysis group at Chalmers University of Technology in Sweden, and two departments of Umicore – Automotive Catalysts at Umicore AG & CO KG (Germany) and Umicore Denmark ApS – are full network partners.
This constellation ensures world-leading competence within catalyst characterisation and testing, computational catalysis and modelling of catalysts behaviour. We are educating four early-stage researchers in the context of a doctoral training network with a strong, multi-faceted emphasis on world leading research, industrial innovation and entrepreneurship.
Developing models to understand catalytic performance
The CHASS project aims to generate knowledge, and enhance the performance, of Cu-zeolite materials for the abatement of NOx by NH3-SCR.
The ultimate objective is to develop a kinetic model(s) for activity, deactivation, and performance of Cu-zeolites for NH3-SCR, including the effects of sulphur oxides and hydrothermal aging, based on atomistic first principles data, applicable for commercial exhaust systems.
In the automotive industry, there is a clear demand for such models from the engine and car manufacturers, as it will most probably become the industry standard to have sufficient (modelling) tools to describe the impact of SO2 on the performance of Cu-CHA catalysts for NH3-SCR.
The CHASS project’s current focus on Cu-CHA catalysts
The model we are currently implementing will be based on the outcome of a combination of quantum-chemical calculations with advanced spectroscopic methods and kinetic measurements. At this stage, we are separately addressing deactivation by sulphur oxides poisoning and that caused by hydrothermal aging.
Our focus is on understanding the poisoning of Cu-CHA catalysts by SO2 at different operating conditions at the atomic level. Using density functional theory (DFT) we are investigating on an atomic scale how sulphur dioxide deactivates Cu-CHA during low-temperature NH3-SCR reaction conditions.
We have calculated the reaction of sulphur dioxide with intermediates suggested to be present during low-temperature NH3-SCR – particularly the peroxo diamino dicopper(II) complex, believed to be a key intermediate in the reaction.
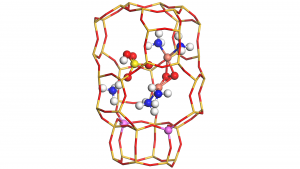
This complex inside the chabazite cage is represented in Fig.1, with the following atomic colour codes: H (white), N (blue), O (red), S (yellow), Cu (bronze), Si (dark yellow sticks) and Al (pink).

Results and conclusions drawn from the study
Our calculations show that sulphur dioxide reacts strongly with this complex, thus suggesting that this reaction is responsible for the deactivation forming tetraamino dicopper(II) sulphate.
We studied the interaction of tetraamino dicopper(II) sulphate with NO and ammonia, two molecules present during NH3-SCR, finally yielding ammonium sulphate and ammonium hydrogen sulphate (represented in Fig.1), causing a physical blocking of the reaction.
The results by DFT calculations suggest that the key mechanism for low-temperature sulphur dioxide deactivation is of physical origin and that the catalyst can be regenerated by exposure to high temperatures where ammonium hydrogen sulphate decomposes.
The reaction of sulphur dioxide with Cu-CHA catalysts pre-treated in different conditions has been followed in situ by X-ray absorption spectroscopy (XAS), Diffuse Reflectance UV-Vis, Infrared and ex situ Raman spectroscopies. XAS and UV-Vis show that sulphur dioxide preferentially interacts with the peroxo diamino dicopper(II) complex, causing a breaking of the dimeric complex and a partial reduction from Cu(II) to Cu(I).
Vibrational spectroscopies confirm the formation of ammonium (and/or hydrogen) sulphate, predicted by DFT calculations. However, the in situ spectroscopic study also indicated a chemical effect of sulphur dioxide, proved by changes in the oxidation state of Cu.
The combination of a physical and chemical effect could be the key to explaining the reasons for the observed reversible and irreversible catalyst deactivation, not yet understood in the literature.
Deactivation has been studied by measuring the effect of Cu content and Si/Al ratio of the zeolite catalysts on sulphur dioxide uptake and catalytic activity after formation of the peroxo diamino dicopper(II) complex.
We find that the effect of sulphur dioxide on deactivation diminishes with increasing its uptake. A formalism/method to model the effect of sulphur dioxide on activity has been developed. We have adapted an approach where deactivation is quantified, without any prior assumptions, as proposed earlier for a different reaction.
The proposed formulation is also the basis for a description of the NOx conversion as a function of sulphur dioxide content, which can be implemented in a kinetic model.
This project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie (grant agreement No. 955839).
Please note, this article will also appear in the sixteenth edition of our quarterly publication.





