Often overlooked, rechargeable batteries play an important part in contemporary life, powering small devices like smartphones to larger ones like electric vehicles.
The key to creating sustainable rechargeable batteries is to make them safer, give them a longer life with more charging cycles, and hold their charge longer.
The problem so far is discovering which solid electrolytes offer such potential advantages.
In a step toward that goal, an Osaka Metropolitan University research group led by Assistant Professor Kota Motohashi, Associate Professor Atsushi Sakuda, and Professor Akitoshi Hayashi of the Graduate School of Engineering has developed an electrolyte with high conductivity, formability, and electrochemical stability.
The findings were published in Chemistry of Materials.
Rechargeable batteries must become more sustainable
Rechargeable batteries, also known as secondary cells, are a type of electrical battery that can be charged, discharged into a load, and recharged many times.
Unlike disposable or primary batteries, which are fully charged and discarded after use, rechargeable batteries can be used multiple times, making them more cost-effective and environmentally friendly.
They are more environmentally friendly than disposable ones, as they reduce the number of batteries manufactured and disposed of.
However, these batteries’ production, usage, and disposal have significant environmental implications. Additionally, charging batteries requires energy, which could contribute to greenhouse gas emissions if the electricity used is generated from fossil fuels.
Developing solid-state electrolytes
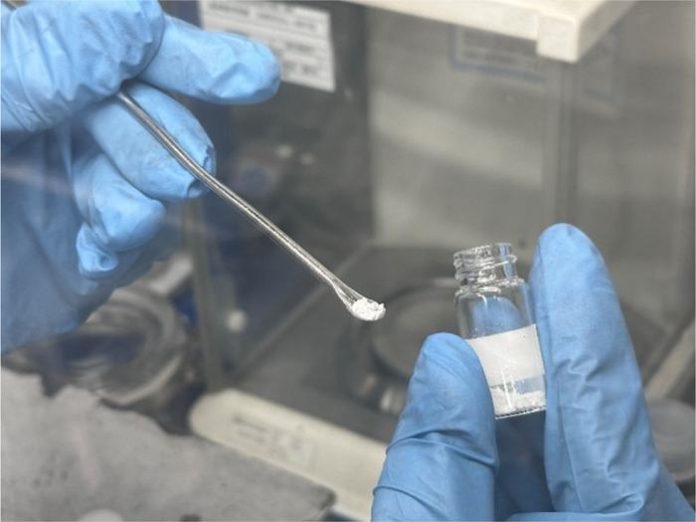
The group achieved high conductivity at room temperature by adding Ta2O5 (tantalum pentoxide) to the previously developed solid electrolyte NaTaCl6, a combination of tantalum chloride and sodium chloride.
The discovered solid electrolyte, Na2.25TaCl4.75O1.25, also has a higher electrochemical stability than conventional chlorides and superior mechanical properties.
“The results of this research are expected to make a significant contribution to the development of composite solid electrolytes, in addition to the glass and crystal solid electrolytes that have been developed to date,” Professor Motohashi stated.
“We will now be focusing on elucidating the ionic conduction mechanism of composite solid electrolytes and further developing materials.”