Phage therapy, once overshadowed by the advent of antibiotics, is now emerging as a pivotal player in the battle against antibiotic-resistant bacteria.
This therapeutic approach harnesses the natural predatory capabilities of bacteriophages, offering a targeted method to eradicate specific bacterial strains. Recent breakthroughs extend its potential beyond traditional infections, encompassing the challenging domains of biofilm disruption and intracellular pathogen treatment.
Moreover, the exploration of phage applications in oncology and innovative drug delivery systems presents intriguing possibilities. As research continues to unravel its complexities and regulatory hurdles, what lies ahead for this once-forgotten therapy?
Historical background of phage therapy
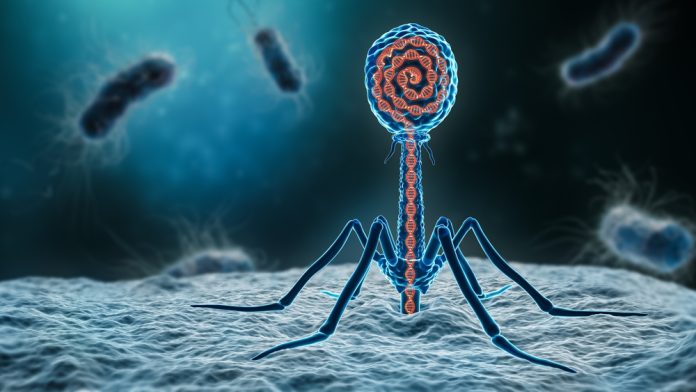
Phage therapy emerged in the early 20th century, taking root with the discovery of bacteriophages by Frederick Twort in 1915 and Félix d’Hérelle in 1917.
This pioneering period marked the inception of utilising bacteriophages – viruses that infect and destroy bacteria – as a therapeutic approach to combat bacterial infections.
The initial enthusiasm surrounding phage therapy was driven by its potential to offer a targeted method of bacterial eradication, distinct from the broad-spectrum nature of chemical treatments.
However, the advent of antibiotics in the 1940s led to a decline in phage research and application. Antibiotics were perceived as a more straightforward and universally applicable solution to bacterial infections, overshadowing the potential of phage therapy.
This shift in focus marked a significant historical development in the medical field, as the promise of antibiotics led to widespread adoption, relegating phage therapy to a secondary role.
The resurgence of interest in phage therapy in recent decades is closely tied to the alarming increase in antibiotic-resistant bacteria. As these multidrug-resistant strains pose a growing threat to public health, the medical community has revisited the potential of bacteriophages as a viable alternative.
This renewed focus highlights the cyclical nature of phage therapy’s prominence, underscoring the importance of historical developments in shaping current research and applications.
Understanding the historical background of phage therapy is essential for appreciating its resurgence and potential to revolutionise modern medicine, offering a targeted strategy against formidable bacterial adversaries.
Phage mechanisms
Understanding the mechanisms by which bacteriophages operate is crucial for advancing phage therapy as a strategic treatment against bacterial infections.
Central to phage mechanisms is the ability of these viruses to target specific bacterial strains. This specificity arises from the interaction between phages and the surface receptors present in bacterial cells.
Upon attachment, phages inject their genetic material into the bacterial host, initiating a process that hijacks the bacterial cell’s machinery for replicating new phage particles.
Once inside the bacterial cell, the genetic material of the phage commandeers the host’s resources to synthesise phage components. This strategic takeover ultimately leads to the assembly of numerous phage progeny within the host.
As the cycle reaches completion, the bacterial cell is lysed or burst, releasing a new generation of phages into the environment, ready to infect additional bacterial targets. This lytic process underscores the efficiency of phage mechanisms in rapidly diminishing bacterial populations.
Phage therapy leverages these mechanisms to develop targeted treatments, offering a precise approach that minimises the disruption to the body’s beneficial microbiota.
The high specificity of phages not only curtails collateral damage but also enhances the therapeutic potential against antibiotic-resistant bacteria.
Combatting antibiotic resistance
In the battle against antibiotic-resistant infections, there exists immense potential to use the natural capabilities of bacteriophages. Phage therapy emerges as a promising strategy against formidable bacterial foes such as MRSA, VRE, and CRE.
These infections have shown resilience against conventional antibiotics, prompting a need for innovative solutions. Engineered phages are skilled at targeting specific bacteria, providing a tailored assault on antibiotic-resistant strains.
By integrating phage therapy with traditional antibiotics, researchers have observed a reduction in bacterial burden, suggesting a synergistic relationship that could revolutionise treatment protocols.
The investigation of phage therapy parallels the precision seen in nuclear physics articles, where meticulous attention to detail and targeted approaches yield significant breakthroughs.
Much like particle physics, where understanding fundamental interactions leads to greater control over atomic nuclei, phage therapy explores the intricate dynamics of bacterial eradication.
This involves techniques similar to mass spectrometry, allowing for precise identification and targeting of bacterial strains.
Phage therapy’s role in addressing Antimicrobial Resistance (AMR) cannot be overstated. In clinical studies, phages demonstrate efficacy against drug-resistant bacteria, highlighting their potential as a pivotal tool in the fight against superbugs.
This potential is similar to the transformative energy harnessed in scientific advancements, offering a powerful alternative to combatting resistance.
By continuing to refine and understand phage therapy, the medical community can make significant strides in overcoming antibiotic resistance challenges, ultimately leading to more effective treatments and improved patient outcomes.
Biofilm targeting strategies
Addressing the formidable challenge of antibiotic-resistant infections extends beyond targeting free-floating bacteria to confronting biofilms, which are complex aggregations of bacteria that exhibit heightened resistance to conventional antibiotics.
These resilient bacterial communities pose significant obstacles in treating chronic infections, often rendering traditional antibiotics ineffective. Phage therapy emerges as a promising biofilm targeting strategy, leveraging bacteriophages’ unique ability to penetrate and disrupt these protective structures.
Phages are viruses that specifically infect bacteria, and their natural propensity to invade biofilm-embedded bacterial communities offers a targeted approach to eradicating stubborn infections.
Unlike antibiotics, which often struggle to penetrate biofilms, phages can diffuse into these matrices, selectively targeting and lysing the bacteria within. Recent research underscores the capability of phage therapy to degrade and disperse biofilms, providing a viable solution to combat chronic infections linked to antibiotic resistance.
The specificity of phages to their bacterial hosts is a pivotal factor in overcoming biofilm-associated challenges. By honing in on the bacteria residing within these biofilms, phage therapy minimises collateral damage to beneficial microbiota, a significant advantage over broad-spectrum antibiotics.
The potential applications of phage therapy in medical and industrial settings are vast, offering innovative solutions to persistent bacterial infections.
The exploration of biofilm targeting strategies through phage therapy continues to advance, promising transformative impacts on infection management.
Intracellular infection solutions
While traditional treatments often struggle to reach and eliminate intracellular pathogens, phage therapy offers a promising solution to this persistent challenge.
Intracellular bacteria, which reside within host cells, present significant treatment hurdles due to the protective environment provided by the host. However, the specificity of phage therapy has emerged as a novel approach to overcoming these obstacles.
Phages possess the unique ability to penetrate host cells and accurately target intracellular bacteria, offering a more refined method compared to conventional antibiotics.
Current research highlights the effectiveness of phage therapy in combating intracellular bacterial infections, underscoring its potential to act where antibiotics may fail.
The specificity inherent in phage therapy enables targeted therapies that can distinguish between pathogenic bacteria and beneficial microbiota, thereby minimising collateral damage to the host’s natural bacterial flora.
This precision is vital in treating infections caused by intracellular pathogens, which often exhibit resistance to standard treatments.
Understanding the interactions between phages, bacteria, and host cells is essential in advancing phage-based therapies. Studies have shown that phages can infiltrate host cells and exert their antibacterial effects directly within the intracellular environment. This capability is crucial in developing therapies that can effectively eradicate intracellular infections without harming the host.
Phage-based drug development continues to expand, with ongoing efforts to refine and optimise these therapies for clinical applications.
As research progresses, phage therapy is poised to become an essential component of modern medicine, particularly in addressing the complex challenges posed by intracellular bacteria.
Innovations in phage vaccines
Amidst the ongoing advancements in medical science, phage vaccines emerge as a pioneering frontier in the battle against bacterial infections.
These vaccines harness bacteriophages to target specific bacterial strains, offering an innovative means of immunisation.
Unlike traditional vaccines, phage vaccines utilise the unique capabilities of bacteriophages to stimulate the immune system directly against pathogenic bacteria, thereby offering a targeted approach to combating bacterial infections.
Phage display technology plays an essential role in the development of these vaccines. This technology allows for the presentation of antigenic peptides on the surface of phages, effectively creating a robust platform for vaccine design.
By leveraging the specificity of bacteriophages, phage vaccines can potentially offer precise immunisation strategies that minimise unintended effects on the body’s beneficial bacteria.
Current research highlights several promising advancements in phage vaccine development, suggesting their potential to revolutionise the prevention of bacterial diseases.
These innovations not only provide a novel approach to immunisation but also hold significant promise in addressing the growing concern of antibiotic resistance.
As the research community continues to explore these innovations, phage vaccines may soon become a cornerstone in our defence against challenging bacterial threats.
Oncological applications
In the field of cancer treatment, phage therapy emerges as a promising frontier, offering innovative strategies for targeting cancer cells with precision.
Unlike traditional methods, phage therapy utilises bacteriophages to specifically target cancer cells, thereby potentially revolutionising oncological approaches.
This precision minimises collateral damage to healthy tissues, a significant advantage over conventional chemotherapy and radiation treatments.
By honing in on the ‘nuclear matter’ of cancer cells, phages can deliver anti-cancer agents directly to tumours, creating new composites of therapeutic solutions that enhance treatment efficacy.
Research into phage therapy has exposed its potential to engage with the immune system, bolstering immune responses against cancer cells.
This dual function not only attacks cancer at its core but also fortifies the body’s natural defences. The specificity of phages in recognising and binding to cancer cell markers can be likened to the principles of quantum entanglement, wherein precise targeting leads to significant reactions at a cellular level, amplifying the therapeutic effects.
The development of phage-based therapies further considers the particle size on epoxy resin, ensuring optimised delivery systems that maximise the therapeutic potential of these agents.
Such advancements pave the way for personalised cancer treatment strategies tailored to the unique genetic and molecular profile of each patient’s cancer.
As ongoing studies continue to refine these applications, phage therapy stands poised to transform not only how we perceive cancer treatment but also how we approach complex medical challenges with cutting-edge technology.
Drug delivery potential
The versatility of phage therapy extends into its potential as a sophisticated drug delivery system, offering a targeted approach for the administration of therapeutic agents.
This innovative method allows for the engineering of phages to carry precise drug payloads directly to specific bacterial targets, which enhances the precision of treatment.
By leveraging the natural targeting capabilities of phages, researchers can design phage-based drug delivery systems that minimise off-target effects and maximise therapeutic efficacy.
Phage therapy’s ability to deliver genetic material or therapeutic compounds to bacterial hosts is a promising avenue of research. This approach not only holds potential for combating antibiotic-resistant infections but also opens new possibilities for treating a variety of bacterial diseases.
The specificity and adaptability of phages make them ideal vectors for delivering therapeutic agents directly to the site of infection, thereby reducing systemic side effects and improving patient outcomes.
Phage-based drug delivery systems, therefore, represent a cutting-edge approach in modern medicine, promising to enhance the efficacy and safety of therapeutic interventions.
Regulatory and safety insights
Building on the promising potential of phage-based drug delivery systems, understanding regulatory and safety insights becomes essential for the successful integration of phage therapy into mainstream medicine.
Regulatory frameworks play a pivotal role in shaping the development and acceptance of phage therapy. These frameworks influence how phage treatments are developed, tested, and implemented, affecting their progress within medical practices.
The establishment of effective regulatory guidelines is necessary to guarantee the safety and efficacy of phage therapy, particularly as it positions itself as a viable alternative to traditional antibiotics.
Central to achieving regulatory compliance are standardised purification processes. These processes are necessary for personalised phage therapy, ensuring that phage preparations are safe and effective for patient use.
The challenge lies in manoeuvring the intricate pharmaceutical regulations that govern these processes. Current infrastructure limitations pose additional hurdles, highlighting the need for collaboration with regulatory agencies to streamline development and approval pathways.
Addressing safety concerns is crucial for the widespread adoption of phage therapy. Historical setbacks, such as the decline in phage research due to the rise of antibiotics, underscore the importance of overcoming these challenges.
Establishing thorough safety protocols is essential to mitigate any risks associated with phage therapy. Collaborating with regulatory agencies can facilitate the creation of these protocols, ensuring that phage therapy meets the strict requirements necessary for clinical application.
Ultimately, overcoming regulatory hurdles and addressing safety concerns through collaborative efforts will be vital in advancing phage therapy as a mainstream solution to combat antibiotic resistance.
Future research directions
As phage therapy continues to emerge as a promising solution to antibiotic resistance, future research directions are focused on enhancing its clinical effectiveness and safety.
A pivotal aspect of this research involves optimising phage mixtures to effectively target antibiotic-resistant bacteria.
This necessitates a profound understanding of phage-bacteria interactions at the molecular level, similar to the precision seen in nuclear physics, where insights into particle yields and interactions inform advancements in other scientific fields.
Future investigations will explore innovative phage delivery systems and their pharmacokinetics to guarantee precise targeting and eradication of bacteria in various states of matter, such as tissues and bodily fluids.
Artificial intelligence is poised to play a crucial role in this domain by analysing complex datasets to predict phage effectiveness and streamline the development of customised therapies.
Additionally, comprehending phage-host interactions and the mechanisms of resistance will provide invaluable guidance for developing new phage-based treatments, ensuring that these therapies remain effective over time.
To facilitate the smooth integration of phage therapy into mainstream medical practice, collaborative efforts are essential.
This includes partnerships between researchers, clinicians, and regulatory bodies, such as the Department of Energy, which can offer the necessary resources and infrastructure for large-scale studies and innovations.
These collaborations will drive the adoption of phage therapy into viable medical solutions for combating antimicrobial resistance.