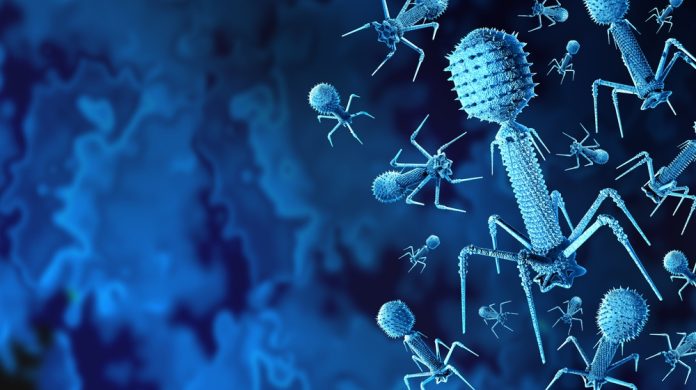
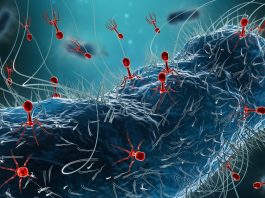
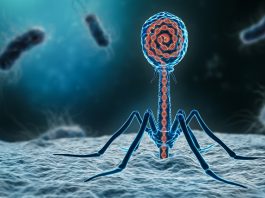
Phage therapy offers a revolutionary and highly targeted solution to the escalating global antimicrobial resistance (AMR) crisis.
By leveraging the natural ability of bacteriophages to target and destroy harmful bacteria, this innovative approach has emerged as a beacon of hope in the fight against multidrug-resistant strains.
As the global community grapples with the growing threat of antibiotic resistance, phage therapy presents a nuanced and adaptable strategy to address the challenges posed by bacterial infections in a rapidly evolving microbial landscape.
How phage therapy works
Phage therapy is an advanced and precise treatment that employs bacteriophages – viruses that specifically infect and kill bacteria – to combat bacterial infections.
Unlike broad-spectrum antibiotics, which often harm beneficial bacteria along with pathogens, phages are highly specific in their action.
This specificity minimises collateral damage to the body’s microbiome, preserving the natural balance of beneficial microorganisms that play a vital role in human health.
In a phage therapy regimen, bacteriophages are carefully matched to the specific bacteria causing an infection. Once administered, the phages bind to the bacterial cells, injecting their genetic material and replicating within the host bacteria.
As the infection cycle progresses, the bacterial cells burst, releasing new phages to attack additional bacterial targets. This self-propagating mechanism amplifies the therapy’s effectiveness and underscores its potential as a personalised and dynamic approach to infection treatment.
Phage therapy’s precision and adaptability make it a promising alternative to traditional antibiotics, particularly in an era marked by increasing resistance to these conventional treatments.
The rising threat of superbugs: Why antibiotics are losing the battle
The rise of antimicrobial resistance poses a critical challenge to modern medicine. Over the past few decades, the widespread overuse and misuse of antibiotics in healthcare, agriculture, and even household products have contributed to the emergence of multidrug-resistant bacteria.
These ‘superbugs’ are capable of outsmarting conventional antibiotics, leaving healthcare providers with limited options to treat infections.
Prominent examples of these superbugs include Methicillin-resistant Staphylococcus aureus (MRSA) and Carbapenem-resistant Enterobacteriaceae (CRE).
These pathogens have developed sophisticated mechanisms to evade even the most potent antibiotics, such as altering their cell structures or producing enzymes that degrade antibiotic compounds.
The implications of this crisis are dire. Without effective antibiotics, routine medical procedures, such as surgeries or chemotherapy, become significantly riskier due to the heightened threat of untreatable infections.
Furthermore, common illnesses could once again become life-threatening, reversing decades of progress in public health.
The urgency to find alternative solutions to antibiotics is clear. Phage therapy, with its targeted action and potential to circumvent resistance, represents a crucial step forward in addressing this global health crisis.
Phage therapy vs antibiotics: A comparative perspective
While antibiotics have been a cornerstone of modern medicine since their discovery, their limitations are becoming increasingly apparent.
Antibiotics typically function by disrupting vital bacterial processes, such as cell wall synthesis or protein production. However, their broad-spectrum nature means they often kill beneficial bacteria along with pathogens, leading to disruptions in the microbiome.
Phage therapy offers a stark contrast. Its specificity allows it to target only the harmful bacteria causing an infection, sparing the beneficial bacteria that support overall health.
This precision reduces the risk of secondary infections, such as Clostridioides difficile, which can result from antibiotic-induced microbiome imbalances.
Another advantage of phage therapy lies in its adaptability. Unlike antibiotics, to which bacteria can develop resistance over time, phages evolve alongside their bacterial hosts. This co-evolutionary relationship makes it harder for bacteria to develop lasting resistance to phages.
By integrating phage therapy into infection control strategies, healthcare providers can not only combat existing infections but also reduce the likelihood of future resistance.
Personalised medicine: A key pillar of phage therapy
One of the most promising aspects of phage therapy is its alignment with the principles of personalised medicine. In this approach, treatments are tailored to the unique characteristics of an individual’s infection.
To administer phage therapy, healthcare providers begin by isolating the bacteria causing the infection and identifying phages that are effective against it.
This targeted process ensures that the treatment is precisely matched to the patient’s needs, maximising efficacy while mitigating unintended effects on the microbiome.
Personalised medicine also allows for dynamic adjustments throughout the treatment process. As bacterial populations evolve, healthcare providers can modify phage cocktails to address emerging resistance.
This adaptability is a cornerstone of phage therapy’s potential to deliver superior outcomes compared to static antibiotic regimens.
Real-world applications and success stories
Phage therapy has already demonstrated its potential in real-world scenarios. In clinical settings, it has been used to treat severe infections caused by antibiotic-resistant bacteria, including Pseudomonas aeruginosa and Staphylococcus aureus.
For patients with chronic infections or those unresponsive to conventional treatments, phage therapy has often served as a life-saving option.
Veterinary medicine has also embraced phage therapy. By treating bacterial infections in livestock, it not only improves animal health but also reduces the reliance on antibiotics in agriculture – a critical step in curbing the spread of resistance.
These applications highlight the versatility and promise of phage therapy across diverse domains, from human healthcare to agriculture.
Regulatory and technical hurdles
Despite its promise, the widespread implementation of phage therapy faces significant hurdles. Regulatory frameworks for phage therapy are underdeveloped, often leading to delays in approvals and uncertainty regarding safety standards.
Establishing standardised protocols for phage isolation, purification, and quality control is essential to ensure consistency and efficacy.
On the technical front, challenges include optimising phage cocktails to address the diversity of bacterial strains and developing rapid diagnostic tools to identify suitable phages for individual patients.
Collaboration among researchers, regulators, and industry stakeholders is critical to overcoming these obstacles and unlocking the full potential of phage therapy.
The future of phage therapy
Looking ahead, the potential of phage therapy to revolutionise infection treatment is immense. As personalised medicine and precision therapies gain traction, phage therapy aligns seamlessly with this future-oriented vision of healthcare.
Imagine a world where tailored bacteriophage treatments target specific pathogens with pinpoint accuracy, minimising collateral damage and reducing the risk of resistance.
This future not only holds promise for treating infections but also for transforming the broader approach to managing microbial threats.
Phage therapy represents a groundbreaking solution to the global antimicrobial resistance crisis. By harnessing the precision and adaptability of bacteriophages, this innovative treatment offers a viable alternative to antibiotics, addressing the urgent need for effective infection control strategies.
With continued research, investment, and collaboration, phage therapy has the potential to reshape healthcare, safeguard public health, and usher in a new era of personalised, targeted treatments.