Dr Jochen Salber, Head of the Department of Experimental Surgery at University Medical Centre Knappschaftskrankenhaus Bochum, discusses the challenges posed by antimicrobial resistance.
At a time when the SARS-CoV-2 virus infection is spreading worldwide, some of us are beginning to realise what social and economic consequences an infection disease can have. At the same time, this infectious organic structure presents us with social and logistical challenges rather than medical-therapeutic ones. Almost all social elites seem to be repressing the fact that mankind is facing another very slowly arising threat – the antimicrobial resistance crisis.
The development of bacterial resistance against antibiotics is an evolutionary survival process of bacteria. During the late 1930s, Chain and Florey from Oxford University began to work on penicillin as part of a comprehensive research programme on antibacterial substances. Since then, antibiotics have saved countless patients lives. However, it has also been a constant head-to-head race between bacterial resistance development and the development of new drugs.1
From the 1960s to the late 1980s, the arsenal of effective antibiotics was filled by the work of many pharmaceutical companies. In particular, this led to the extraordinary success of medicine and many of its therapies, especially surgical and interventional therapies. However, antimicrobial resistance is ancient and the resistome (a term for all of the antibiotic resistance genes and their precursors in both pathogenic and non-pathogenic bacteria) is a highly dynamic and steadily increasing challenge.2 Therefore, all of the modern societies should have known that a drop in antibiotic research, or even an end, would result in them potentially losing the race.
One of the reasons contributing to the antimicrobial resistance crisis, is that for almost a decade, many big pharmaceutical companies such as Bristol-Myers Squibb and Abbott, have withdrawn from any highly expensive and non-economically reimbursed antibiotic research and development. However, this is only one of the causing factors – summarised in Table 1 above are the different causes of these non-judicious behaviours that have led to the global resistome and antimicrobial resistance crisis.

The antimicrobial resistance crisis in numbers
It is still a huge challenge to evaluate the exact economic impact of resistant bacterial infections globally, but some data from the US and European Union have given us a rough impression of how substantial the socio-economic burden actually is. Every year in the US, 99,000 patients die based on antibiotic-resistant pathogen-associated hospital-acquired infections (HAIs). In 2006, 50,000 patients died due to two common HAIs (pneumonia and sepsis), and as a result the economic burden was around $8b.3
Hundreds of thousands of people worldwide (approximately 33,000 in the EU) die every year from the consequences of infections with resistant bacteria. The United Nations warns that deaths will skyrocket if this is not acted on immediately. According to this, 10 million people could die from resistant bacteria every year by 2050, more than cancer today.4
The consequences of the antimicrobial resistance crisis for modern medicine
All achievements of modern medicine are only possible through the application of medical devices (MD). This is especially true for class three invasive medical devices according to the European Union’s medical device regulations (MDR). Annually, around 180,000 pacemakers, 260,000 cardiovascular stents, 200,000 hernia meshes, 365,000 hip and knee endoprostheses are implanted into patients in Germany. Furthermore, millions of central venous catheters, ports, urinary catheters, and wound dressings for example are used invasively.5
All of these implants are made of biomaterials with medical grade approval. Amongst them are different classes of materials, such as metal alloys, ceramics and polymers. Although these biomaterials are mainly described as ‘inert’ (they do not harm the host’s biology), or bio-active to support the host’s biology in different ways, they still remain as foreign materials. These foreign materials possess the disadvantage that bacteria and commensals of the host and pathogens interact with these biomaterials in a severely dangerous manner. Bacteria can form biofilms on the outer and inner surfaces of such biomaterials, often avoiding the host’s immune defence or applied antibiotics if the patient is suffering from an infection.6
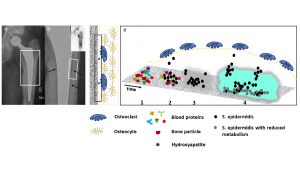
The biofilm as essential survival strategy of bacteria
After implantation of a medical device (such as an orthopaedic endoprosthesis), a competition between bacterial colonisation and tissue integration takes place to conquer the surface of the implant. This phenomenon was first described by Gristina et al in 1988.7 Since then, numerous specialist articles on this topic have been published.8,9 To simplify matters, the example of the endoprosthesis remains, whereby the basic mechanistic and pathophysiological principles can be applied to any invasive implant. Fig.1 shows a late periprosthetic joint infection (PJI) of a male patient with increasing problems one year after a primary total hip arthroplasty on the left side.6
As soon as bacteria come into contact with such implant surfaces, they adhere and colonise. During the first 48 hours, the biofilm is in its juvenile stadium and a few antibiotic strategies are still working. However, once the biofilm has matured, these reserve antibiotic strategies will no longer work, and the last resort will be removing the implant.
Is the development of novel antibiotics the only solution?
The answer to this question is no, not at all. Scientists are working globally on alternative strategies to conventional antibiotics, such as antibodies, probiotics, bacteriophages, antimicrobial peptides, biofilm-preventing and disrupting enzymes, and last but not least antimicrobial implant surface modifcations.10 Nevertheless, without effective antibiotics and alternative, complementary antimicrobial strategies, the success of major surgery and interventional therapies, temporarily applied medical devices or implanted mid- and long-term devices would be strongly compromised.
Modern medicine would become a mission impossible!
References
- Ventola, C.L. 2015. The Antibiotic Resistance Crisis. Part 1: Causes and Threats. P&T. 2015;40(4):277-283
- Wright, G.D. 2007. The antibiotic resistome: the nexus of chemical and genetic diversity. Nature Reviews Microbiology. 2007;5(3):175–186
- Baloch, Z. et al. 2018. Antibiotic resistance: a rundown of a global crisis. Infect Drug Resist. 2018;11:1645-1658
- Baars, C.H., and Lambrecht, O. 2019. Development of Antibiotics – A disaster with an announcement. Available at: https://www.tagesschau.de/investigativ/ndr/antibiotika-pharmakonzerne/
- Salber,J., and Viebahn, R. 2017. Biocompatibility of antimicrobial polymeric materials for medical applications – How much in vitro is mandatory, how much is sufficient? eCM Meeting Abstracts, TERMIS EU 2017; Collection 2/0159:161
- Idrees, A. et al. 2018. Drug-free antibacterial polymers for biomedical applications. Biomedical Science and Engineering – PAGEPress. 2018;2(1):1-12. www.pagepress.org/technology/index.php/bse/article/view/39
- Gristina, A.G., Naylor, P., and Myrvik,Q. 1988. Infections from biomaterials and implants: a race for the surface. Med Prog Technol. 1988;14(3-4):205-24
- Wagner, C., and Hänsch, G.M. 2015. Pathophysiology of implant-associated infections. From biofilm to osteolysis and septic loosening. Orthopäde. 2015;44:967–973
- Zimmerli, W., and Sendi, P. 2017. Orthopaedic biofilm infections. APMIS 2017;125:353–364
- Ghosh, C., Sarkar P., and Issa, R. et al. 2019. Alternatives to Conventional Antibiotics in the Era of Antimicrobial Resistance. Trends Microbiol. 2019;27(4):323-338
 The author was supported by the HyMedPoly project. HyMedPoly received funding from European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska -Curie grant agreement No. 643050.
The author was supported by the HyMedPoly project. HyMedPoly received funding from European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska -Curie grant agreement No. 643050.
Dr Jochen Salber (PhD, MD)
Senior Physician Surgery
Head of the Department of Experimental Surgery
Department of Surgery
University Medical Centre Knappschaftskrankenhaus Bochum
Hospital of the RUHR-University Bochum
+49 234 32 21819
jochen.salber@rub.de
www.kk-bochum.de
www.researchgate.net/lab/Jochen-Salber-Lab
Please note, this article will also appear in the second edition of our new quarterly publication.