The Nano4Tarmed consortium aims to develop 2D nanoplatforms for active drug delivery, diagnosis and treatment of osteosarcoma.
Osteosarcoma, a primary malignant bone tumour, poses a significant challenge in oncology due to its complex pathogenesis and often aggressive nature. Osteosarcoma primarily affects the long bones of adolescents and young adults, thus presenting unique clinical and therapeutic considerations.
It accounts for approximately 5% of childhood tumours. More than 50% of these tumours in children and adolescents arise from the long bones around the knee. Osteosarcoma is rarely seen in soft tissue or visceral organs, and there appears to be no difference in presenting symptoms, tumour location, and outcome for younger patients (<12 years) compared with adolescents.
As the most common primary bone cancer in children and adolescents, understanding its complexity and evolving treatment strategies is paramount for improving young patient outcomes and their quality of life.
Treatment of osteosarcoma
Successful osteosarcoma treatment generally requires the combination of effective systemic chemotherapy and complete resection of all clinically detectable diseases. Patients with proven or suspected osteosarcoma must first have an evaluation by an orthopaedic oncologist familiar with the surgical management of this disease. This evaluation, which includes imaging studies, should be done before the biopsy because an inappropriately performed biopsy may jeopardise a limb-sparing procedure.
Additionally, protective weight-bearing is recommended for patients with tumours of weight-bearing bones to prevent pathological fractures that could prevent limb-preserving surgery. Randomised clinical trials have proven that both neoadjuvant and adjuvant chemotherapy are effective in preventing relapse in patients with clinically non-metastatic tumours.
Interestingly enough, the Paediatric Oncology Group (POG) conducted a study in which patients were randomly assigned to either immediate amputation or amputation after neoadjuvant therapy. Many patients declined to be assigned randomly, and the study was stopped without approaching the stated accrual goals. In the small number of patients receiving osteosarcoma treatment, there was no difference in outcome between those who received preoperative and postoperative chemotherapy.
Nonetheless, chemotherapy for osteosarcoma is usually performed by administering drugs with dual or triple-modal approaches and mechanisms of action. The major drawback of such approaches lies in the frequent side effects caused by the medicines’ toxicity to healthy cells and the limitations given by possible drug cross-interactions.
All these limitations negatively affect osteosarcoma treatment and, thus, patients’ quality of life. The limitations described can be minimised by applying intelligent drug delivery systems, such as nanoplatforms, which can efficiently deliver multiple drugs to the tumour’s location without affecting healthy cells. These nanoplatforms are usually represented by 2D nanomaterials based on graphene derivatives.
The project Nano4Tarmed, presented here for the first time, aims to develop theranostic approaches based on plasmonic and 2D nanomaterials for detecting and treating cancer diseases.
Here, we would like to summarise the most important results obtained in the framework of the project Nano4Tarmed.
Detection of cancer diseases using plasmonic nanomaterials
Surface-enhanced Raman spectroscopy is an advantageous analytical technique for detecting molecular targets at ultra-low concentrations. It works on the principles of interaction of analytes with plasmonic nanomaterials composed of metals, including silver and gold, where this physicochemical interaction dramatically enhances analytical signals frequently of several orders of magnitude.
Here, we utilised this analytical technique in the multiplex detection of free prostate-specific antigen (fPSA) together with prostate-specific antigen (PSA) in whole human blood for a diagnosis of prostate cancer. It is well known that a currently and solely utilised biomarker, prostate-specific antigen (PSA), presents many benefits and some considerable limitations anchored in the generally high number of false-positive results. Including fPSA in the test set scheme could improve the method’s reliability.
We have developed a novel method utilising magnetic nanocomposites Fe3O4@Ag, and we could detect fPSA and PSA at concentrations of both compounds as low as 0.5 ng/mL with the use of Raman microscopy and magnetic separation of the target compounds from the complex mixture represented by the human whole blood. The following developed method was based on applying ZrO2@Au nanocomposites for detecting gliomas in the fresh human brain tissue obtained during brain surgery.
The combination of gold and zirconium atoms allows us to tune the plasmon frequency, which is one of the critical characteristics of the used plasmonic nanomaterial and a crucial parameter defining the signal enhancement factor. The applied ratio of both metals led to a shift of the plasmon frequency to a region of the used excitation laser (532nm) and maximised the signal enhancement effect.
The developed method can discriminate between peripheral parts of the glioma with lower numbers of cancerous cells and the centre part, which usually holds higher populations of cancerous cells and is often affected by advanced demonstrations, including necrosis.
Development of 2D nanoplatforms for intelligent drug delivery
The development of drug delivery nanoplatforms presents one potentially exciting approach to cancer treatment. This approach allows the targeted drugs to go directly to the tumour site without affecting the surrounding healthy tissues. This approach considerably improves the efficacy of such treatment and, at the same time, minimises unwanted side effects that often negatively affect patients’ health. We focused on developing 2D nanoplatforms using graphene oxide that could deliver functionalised platinum complexes based on cisplatin scaffold, a leading worldwide chemotherapeutic drug.
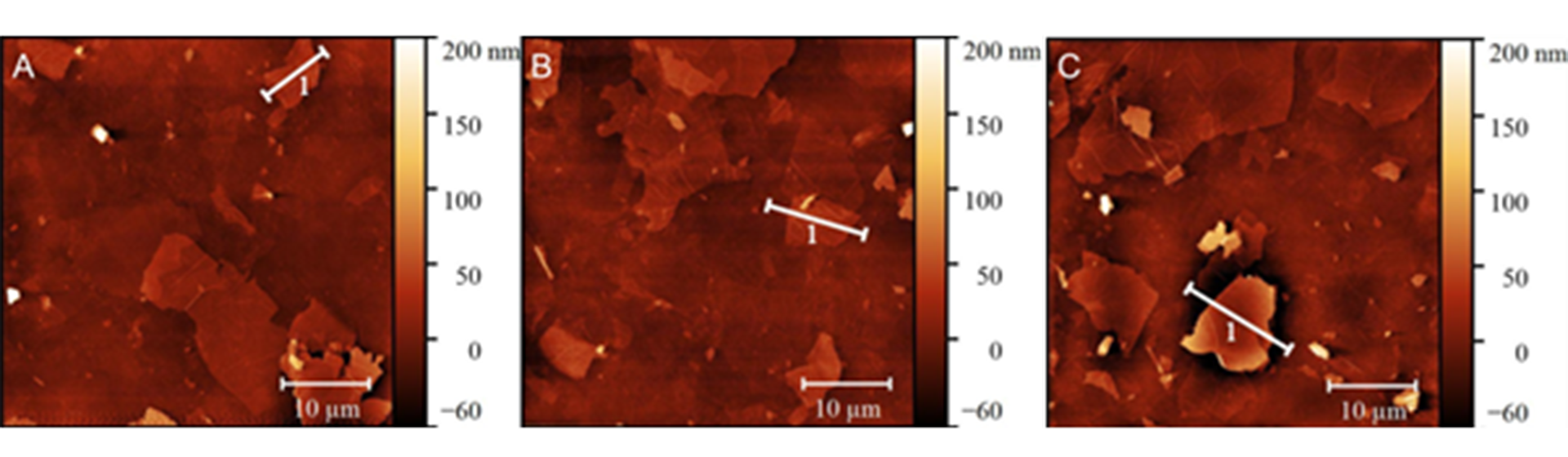
First, we focused on the synthesis of single-drug delivery platforms. The development nanoplatform consisted of the graphene oxide-based substrate functionalised using eight-armed polyethyleneglycol (PEG). The developed nanoplatforms were characterised using various analytical techniques, including atomic force microscopy (AFM; the illustrative results are shown in Fig. 1), scanning electron microscopy and vibrational spectroscopy.
The platinum-based complexes were afterwards anchored covalently on the surface of the platform. Our recent publication (Zarska et al., Nanomaterials, 2022) showed a synergy effect between the nanoplatform and the bound drugs, increasing the therapeutic effect, as demonstrated in Fig. 2. The nanoplatform accomplishes excellent cellular proliferation inhibition in osteosarcoma, strictly related to increased cellular uptake. To a lesser extent, this cellular internalisation was seen in glioblastoma.
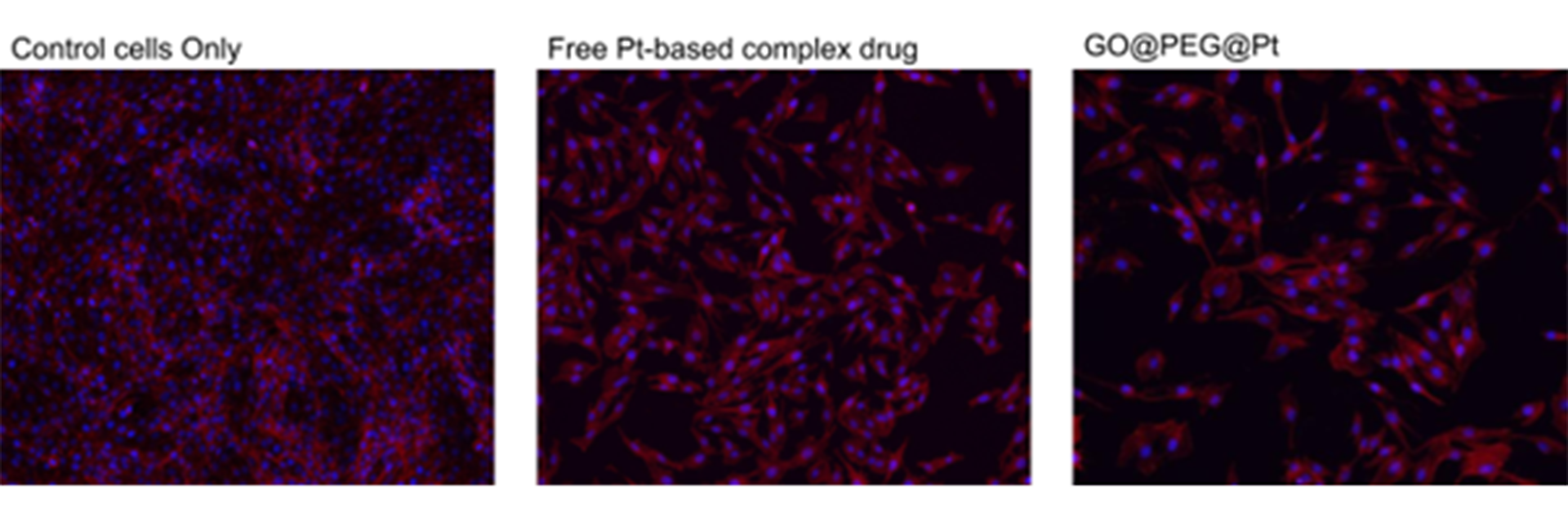
It was also shown that the proposed GO@PEG nanoplatform is promising for inhibiting cell migration and minimising the metastatic process. Thus, the GO@PEG nanoplatform stands as an exciting tool in cancer treatment, with the possibility of targeting diverse types of cancers.
Next, we shifted our focus to developing multi-drug smart-delivery systems, again based on applying graphene oxide. The selected transported drugs were Pt-based complexes, analogous to previous experiments, and Doxorubicin, another approved chemotherapeutic with a different mode of action. Both drugs were bound on the surface of the graphene oxide and functionalised using polyethyleneglycol to introduce chemical anchors for the chemotherapeutic agents. The results indicate that both drugs are delivered inside cancer cells with a considerable therapeutic effect.
Conclusions and future perspectives
The consortium Nano4Tarmed developed a new nanoplatform for the drug delivery of Pt-based and small organic anticancer drugs, including Doxorubicin. The nanoplatform is based on graphene oxide 2D nanomaterial functionalised using eight-branched polyethylene glycol to increase its biocompatibility and loading capacity. The platform now allows the delivery of two distinct drugs with various modes of action to cancer cells such as osteosarcoma, glioma and breast cancer cells.
There is considerable potential for increasing the number of anchored and delivered drugs to three various molecules and further expanding its application potential in cancer treatment. During its first three years, the consortium also developed several nanotechnology-based plasmonic biosensors to detect cancer cells and important cancer biomarkers, including important markers of prostate cancer: PSA and fPSA.
The future perspective aims to combine drug delivery systems with plasmonic-based diagnostic tools to create theranostic platforms to detect cancer cells and deliver drugs directly into their compartments.
Please note, this article will also appear in the 18th edition of our quarterly publication.