Researchers at the University of Manchester outline the role of prominent metabolic dysregulation in age-related neurodegeneration in the human brain – a metabolic basis for dementia with potential therapeutic implications.
In this article, we provide an overview of recent discoveries from our ongoing research programme in neurodegenerative diseases and the associated age-related neurodegeneration and dementia syndromes. ‘Neurodegeneration’ means the progressive loss of structure and function of nervous tissue. ‘Dementia’ is a term that describes a group of conditions in which decline in memory and the ability to learn and understand are sufficient to impair the capacity for independent living.
Age-related dementia: impact
There are several age-related dementia syndromes, the most common of which are caused by Alzheimer’s disease (AD) and vascular disease (vascular dementia (VaD)).1
Dementia overtook heart disease for the first time in 2017 and is now Britain’s biggest killer.2
AD represents the greatest unmet clinical need in neurology, the branch of biology and medicine that deals with the structure and function of nerve cells and the nervous system.
Aims
Our programme at the Centre for Advanced Discovery and Experimental Therapeutics at the University of Manchester aims to identify hitherto unrecognised molecular processes that play central roles in the causation of age-related dementia.
Our objective is to pinpoint new, tractable molecular targets for therapeutic intervention and to contribute to the discovery and ultimately the development of one or more effective new disease-modifying medicines for treating age-related dementias.
At present, we have focused our studies on two neurodegenerative diseases, AD and Huntington’s disease (HD), with the idea that disease processes in the latter can serve as a comparator for those of the former.
We have found, in the forms of dementia caused by AD and HD, that severe metabolic perturbations clearly overlap in respective at-risk brain regions.3-8
Requirement for new approaches
There is a widely agreed need for new approaches towards the experimental therapeutics of age-related dementia, since the existing drug-development pipeline has had few successes and has suffered many failures.9
Current strategies in experimental AD therapeutics have focused mainly on targeting disease-causing aggregation of the proteins Abeta and tau. However, these approaches have so far proven unrewarding as routes to development of effective disease-modifying therapeutic molecules.
A recent study reported that, during a ‘2002 to 2012 observation period, 413 AD trials were performed: 124 Phase 1 trials, 206 Phase 2 trials, and 83 Phase 3 trials’.9
The overall success rate during this observation period was 0.4% (i.e. 99.6% failure rate). The 0.4% of effective drugs included three cholinesterase inhibitors, which provide modest symptomatic relief in those afflicted by mild to moderate AD-related dementia but are not disease-modifying treatments.
Diabetic organ damage can serve as a Rosetta Stone for investigation of age-related dementia
We initiated this programme following our discovery of closely related metabolic perturbations in tissues damaged by Type 2 diabetes (e.g. peripheral nerves) and by AD in the brain.3,7,8,10-14
Multiple lines of evidence strongly link Type 2 diabetes with several of the age-related dementias.15
Diabetes elevates levels of glucose in the blood and organs that are at risk for metabolic damage, for example nervous tissue.13
In addition, we have extended our studies to analyse the effects of neurodegeneration caused by HD in multiple brain regions that display different degrees of damage during disease progression.
HD has a well-established genetic basis, whereas, by contrast, the large majority of AD cases are sporadic.
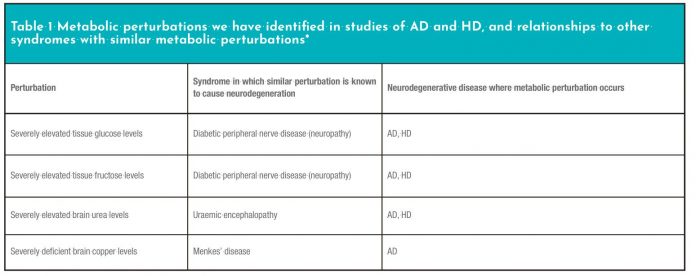
We have found that at least three major metabolic perturbations in HD mirror those in AD (Table 1).5-8,16
Research questions
We are asking two research questions:
- Might the molecular perturbations that cause neurodegeneration in the different forms of age-related dementia mirror those that cause peripheral-nerve damage in diabetes? and
- Could cerebral blood vessel disease in dementia mirror vascular damage in diabetes (termed ‘diabetic vasculopathy’)?
‘Vasculopathy’ is a general term that describes any disease affecting blood vessels whose underlying causative mechanism is usually unknown.
Several strong lines of evidence link VaD to type-2 diabetes.
Diabetes-like vascular damage affecting small arteries (termed ‘arteriosclerosis’ or hardening of the arteries) occurs in the brain in VaD.17
This may also be the case in other age-related neurodegenerative diseases, where disease of small blood vessels (small vessel disease (SVD)) causes increased prevalence of white-matter lesions and foreshadows the onset of dementia.18
Quantitating molecular composition of the brain in neurodegenerative disease
We have developed a five-dimensional, multi-omic process to determine the composition of brain and other nervous tissues and applied it to improve understanding of molecular mechanisms leading to brain damage in dementia.
This multi-omic approach comprises the following:
- Metabolomic analysis by gas chromatography-mass spectrometry (GC-MS; approximate mass range ~70-400 Da);
- Metallomics by inductively coupled plasma mass spectrometry (ICP-MS);
- Metabolomics by liquid chromatography-mass spectrometry (LC-MS; ~200-1,000 Da);
- Proteomics; and
- Transcriptomics.
Here, we have discussed results derived mainly from the first two methods.
Summary of main findings
Our studies have identified severe perturbations in brain levels of four prominent metabolites, in cases of age-related dementia compared with controls (Table 1). Each of these metabolites plays a central role in the pathways of energy metabolism.
Levels of glucose, fructose, urea, and copper are each severely perturbed in AD (Table 1). Three of these perturbations are present in both AD and HD, although their regional distribution varies between the two diseases.6-8 The severity of these perturbations is such that each one is likely to be cytotoxic in its own right, based on comparisons with other known disease entities where similar metabolic perturbations occur. It appears that these defects could well synergise in at-risk brain regions in neurodegeneration to cause characteristic patterns of brain damage. For each metabolite, there is a known syndrome, where perturbations similar to those we have measured in AD and HD cause tissue damage in either brain or other nervous tissue (Table 1).
Replication
A key requirement, before the results of studies are generally accepted, is that independent researchers must replicate the results in other patient populations.
Consistent with this benchmark, a recent study from the National Institute of Aging (US National Institutes of Health) reported elevated brain glucose and evidence for impaired glucose utilisation in AD.19
This study provides important replication of our prior findings.7,8
A separate international group16 has now replicated our earlier findings5,6 concerning elevation of urea, to potentially toxigenic levels, in the brain in HD. This study also provides compelling evidence that elevated brain urea in HD can antedate the development of dementia by many years. It is therefore probable that this perturbation is causative of neurodegeneration and that it could therefore serve as a new target for therapeutic development.
This is an important paradigm shift since elevated brain urea occurs in other contexts where it causes neurodegeneration and where known interventions can lessen its impact.
Implications for experimental therapeutics
Our findings provide new targets for the development of new therapeutic interventions for age-related neurodegeneration.
Specifically, they have the potential to refocus thinking in the field of the experimental therapeutics of age-related dementia, directing attention towards defects in the brain pathways that regulate energy metabolism.
Approaches targeting such pathways differ substantively from methodologies that target protein aggregation.
References
- McKhann GM, et al. The diagnosis of dementia due to Alzheimer’s disease: recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 2011; 7: 263-269. https://www.ncbi.nlm.nih.gov/pubmed/21514250
- Knapton S. Dementia now Britain’s biggest killer, overtaking heart disease for the first time. The Telegraph (London). 7 November 2017. https://www.telegraph.co.uk/science/2017/11/07/dementianowbritainsbiggestkillerovertakingheartdisease/
- Schönberger SJ et al. Proteomic analysis of the brain in Alzheimer’s disease: molecular phenotype of a complex disease process. Proteomics 2001; 1: 1519-1528. https://www.ncbi.nlm.nih.gov/pubmed/11747211
- Schönberger SJ et al. Proteomic analysis of the human brain in Huntington’s disease indicates pathogenesis by molecular processes linked to other neurodegenerative diseases and to type-2 diabetes. J Huntington’s Dis 2013; 2013: 89-99. https://www.ncbi.nlm.nih.gov/pubmed/25063432
- Patassini S et al. Identification of elevated urea as a severe, ubiquitous metabolic defect in the brain of patients with Huntington’s disease. Biochem Biophys Res Commun 2015; 468: 161-166. https://doi.org/10.1016/j.bbrc.2015.10.140
- Patassini S et al. Metabolite mapping reveals severe widespread perturbation of multiple metabolic processes in Huntington’s disease human brain. Biochim Biophys Acta Mol Basis Dis 2016; 1852: 1650-1662. http://dx.doi.org/10.1016/j.bbadis.2016.06.002
- Xu J et al. Elevation of brain glucose and polyol-pathway intermediates with accompanying brain-copper deficiency in patients with Alzheimer’s disease: metabolic basis for dementia. Sci Rep 2016a; 6: 27524. https://www.nature.com/articles/srep27524
- Xu J et al. Graded perturbations of metabolism in multiple regions of human brain in Alzheimer’s disease: Snapshot of a pervasive metabolic disorder. Biochim Biophys Acta Mol Basis Dis 2016b; 1862: 1084-1092. https://www.ncbi.nlm.nih.gov/pubmed/26957286
- Cummings JL et al. Alzheimer’s disease drug-development pipeline: few candidates, frequent failures. Alzheimer’s Res Ther 2014; 6: 37. https://doi.org/10.1186/alzrt269
- Cooper GJS et al. Regeneration of the heart in diabetes by selective copper chelation. Diabetes 2004; 53: 2501-2508. https://doi.org/10.2337/diabetes.53.9.2501
- Cooper GJS et al. Demonstration of a hyperglycemia-driven pathogenic abnormality of copper homeostasis in diabetes and its reversibility by selective chelation. Quantitative comparisons between the biology of copper and eight other nutritionally essential elements in normal and diabetic individuals. Diabetes 2005; 54: 1468-1476. https://doi.org/10.2337/diabetes.54.5.1468
- Zhang S et al. Diabetic cardiomyopathy is associated with defective myocellular copper regulation and both defects are rectified by divalent copper chelation. Cardiovasc Diabetol 2014; 13: 100. https://www.ncbi.nlm.nih.gov/pubmed/24927960
- Freeman OJ et al. Metabolic dysfunction is restricted to the sciatic nerve in experimental diabetic neuropathy. Diabetes 2016; 65: 228-238. https://doi.org/10.2337/db15-0835
- Xu J et al. Evidence for widespread, severe brain copper deficiency in Alzheimer’s dementia. Metallomics 2017; 9: 1106-1119. https://www.ncbi.nlm.nih.gov/pubmed/28654115
- Cooper GJS. Therapeutic potential of copper chelation with triethylenetetramine in managing diabetes and Alzheimer’s disease. Drugs 2011; 71: 1281-1320. https://www.ncbi.nlm.nih.gov/pubmed/21770477
- Handley RR et al. Brain urea increase is an early Huntington’s disease pathogenic event observed in a prodromal transgenic sheep model and HD cases. Proc Natl Acad Sci USA 2017; https://www.ncbi.nlm.nih.gov/pubmed/29229845
- Gorelick PB et al. (2011) Vascular contributions to cognitive impairment and dementia: A statement for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2011; 42: 2672–2713. doi:10.1161/STR.0b013e3182299496
- Prins ND et al. Cerebral white matter lesions and the risk of dementia. Arch Neurol 2004; 61: 1531-1543. https://jamanetwork.com/journals/jamaneurology/fullarticle/786829
- An Y et al. Evidence for brain glucose dysregulation in Alzheimer’s disease. Alzheimer’s Dem 2018; 14: 318-329. https://www.ncbi.nlm.nih.gov/pubmed/29055815
Garth J S Cooper, DSc (Oxon), FMedSci
Centre for Advanced Discovery and Experimental Therapeutics
Faculty of Biology, Medicine and Health
School of Medical Sciences
Division of Cardiovascular Sciences
The University of Manchester
garth.cooper@manchester.ac.uk