Microbiology expert Valerie Edwards-Jones provides in-depth insights into the current antimicrobial resistance landscape and effective strategies to combat this urgent health threat.
There is an unprecedented rise in the levels of antimicrobial resistance in common pathogens, but should we really be concerned? In 2019, Antimicrobial resistance (AMR) in common pathogens was directly responsible for 1.27 million deaths globally, with Escherichia coli responsible for the most deaths, followed by K pneumoniae, S aureus, A baumannii, S pneumoniae, and M tuberculosis.1
What is antimicrobial Resistance?
Antimicrobial is a generic term for any compound that has antimicrobial properties, and Figure. 1 demonstrates the general categories of compounds. Antimicrobial resistance is an umbrella term for the loss of effectiveness amongst agents used to fight infections, whether bacteria, viruses, fungi or parasites. While resistance is a natural phenomenon and several organisms are naturally resistant, AMR in common pathogens has been acquired and accelerated by inappropriate use of antimicrobial drugs, poor infection prevention and control practices and a lack of new antimicrobial drugs being developed.
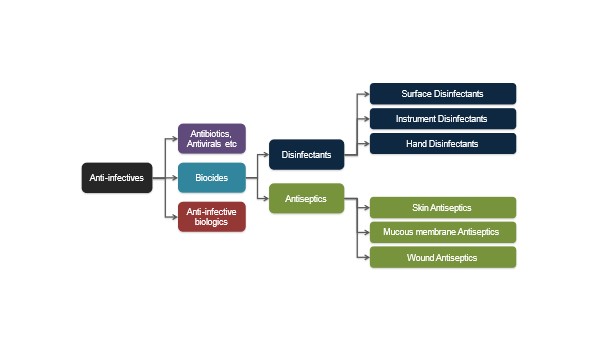
Figure. 1 General Categories of Antimicrobial Compounds
The most concerning development is the loss of susceptibility to common first-line antibiotics used to treat a wide array of pathogenic bacteria such as Staphylococcus aureus, Mycobacteria tuberculosis, Neisseria gonorrhoeae and most recently amongst the common faecal and environmental Gram-negative organisms, such as E.coli, Acinetobacter baumanii or Klebsiella pneumoniae,. 2,3
In addition, pathogens continue to adapt and are developing resistance to some third and fourth-generation antibiotics made available to overcome the resistance problem, for example, carbapenem resistance in Enterobacteriacae (CRE).
AMR has been demonstrated in most categories of antimicrobial compounds but is marked amongst the antibiotics. Some bacterial strains have developed multidrug (antibiotic) resistance (MDR), where the bacterial strain is not just resistant to one class of antibiotic but to several, leaving the healthcare practitioner unable to find an antibiotic to treat the patient with.
Mechanisms of resistance
These MDR strains either develop through step-wise mutation, i.e. developing resistance to one antibiotic, then another, then another, but most commonly, MDR is acquired through plasmid transfer, which then may persist in a bacterium or genes for MDR may integrate into the genome.
The main mechanisms of antimicrobial resistance are (figure 2)
- Limiting the uptake of a drug
- Modification of a drug target site that modifies binding capacity (mutation e.g. gentamicin)
- Inactivation or modification of a drug (usually by secreted enzymes e.g beta-lactamases)
- Active efflux of a drug
- The synthesis of resistant metabolic pathways (e.g. folic acid synthesis and sulphonamides)
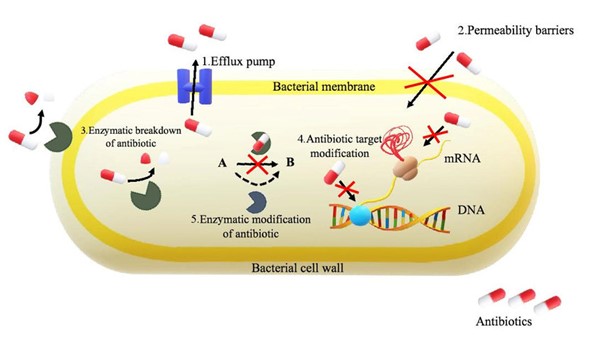
Other molecular mechanisms have been identified in individual organisms, and this demonstrates that they are continually evolving to overcome the effects of antimicrobials and to proliferate in healthcare settings where their use is higher than in the natural environment.
Limited Progress in Antibiotic Development
Alternative antimicrobial agents are being sought. In 2021, it was reported that there were only 43 new antibiotics in the development and clinical trial stage. Of the 43 new antibiotics, 26 are active against WHO AMR pathogens, 12 against Mycobacterium tuberculosis and five against Clostridioides difficle. Going forward, more research funding will be made available for the development of new antibiotics and for non-traditional agents to be used. As of 2021, 27 non-traditional antibacterial agents (nine antibodies, four bacteriophages, and phage-derived enzymes, eight microbiome-modulating agents, two immunomodulating agents and four miscellaneous agents) were being developed as alternative treatments. 4
Because of the success of antibiotics and other antimicrobial agents in resolving infection, we have seen a massive advancement in innovative surgical and medical techniques, with transplants and cancer treatments commonplace around the world. However, if antimicrobial treatments were no longer available due to AMR, then the continuation of such advancements would decline rapidly with a high risk of infection in this group of immunocompromised patients.
In order to mitigate these problems, we have to act now to prevent any further AMR development, prevent its spread, and produce new alternatives with the hope AMR will not develop rapidly in those.
Global strategies to combat AMR
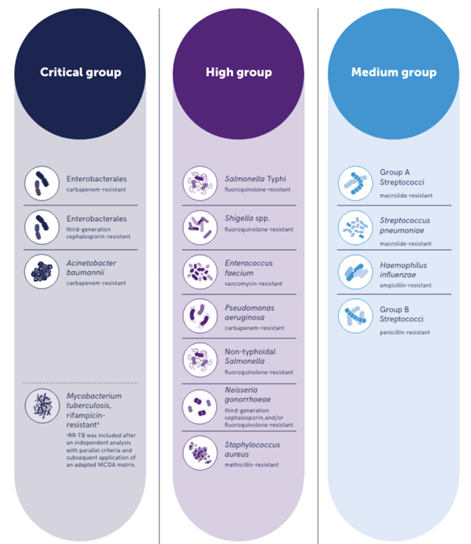
There is a global plan to tackle AMR, and the World Health Organisation (WHO) has identified 15 priority pathogens categorised as critical, high, and medium importance based on those that pose the highest threat to public health due to limited treatment options, disease burden (mortality and morbidity) and where prevention and transmissibility are problematic (See figure 3).2 Patients who succumb to infections associated with these pathogens have few treatment options.2,3.
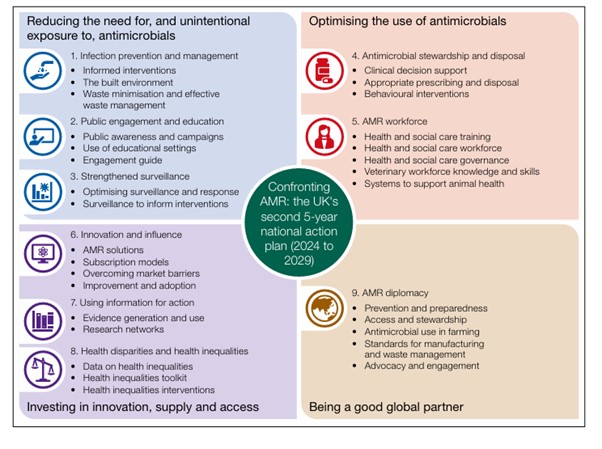
The UK has a 20-year vision for tackling AMR through a series of 5-year National Action Plans (NAPs), which aim to bring together organisations across government to contain, control and mitigate AMR by 2040.5 The most recent plan published on 8th May 2024 commits to restricting the unnecessary use of antimicrobials in humans and animals, strengthening the surveillance of drug-resistant infections, and incentivising industry to develop the next generation of treatments summarised in figure 4.6
How UKHSA is tackling AMR
The UK Health Security Agency (UKHSA) is responsible for monitoring any changes to AMR across the country, overseeing human health surveillance and threat detection, tracking and analysing resistance, developing evidenced-based interventions, implementing targeted public interventions, and embedding infection prevention and control in healthcare settings.
The UKHSA also involves translating research into possible policy outputs and public health campaigns and developing the targets and commitments, including coordinating consensus groups across the four nations of the United Kingdom. Cutting-edge analytical and modelling methods are used to highlight organisms of concern to the national advisory committee (APRHAI) and inform further action.
The Incidents Outbreaks & Stewardship team (IOS) leads investigation and response to outbreaks in health and care settings which involve pathogens with multidrug resistance and develops AMR resources for clinicians6. The UKHSA has also developed high-profile public engagement and awareness communications to ensure the public is engaged in the fight to affect positive change, with initiatives such as the Antibiotic Guardian, and Keep Antibiotics Working campaign. 7,8
General effective measures implemented in healthcare settings to mitigate AMR include Infection prevention of control policies, which minimise the impact of the environment, hygiene and sanitation on infection rate, thus reducing the need for antimicrobials. The implementation of antimicrobial stewardship policies, where antimicrobial usage is done so with adherence to prescribed dosage and treatment options, has been shown to have an impact.
In a recent global systematic review, it was shown that antibiotic stewardship programs were associated with a 10% reduction in antibiotic prescriptions and a 28% reduction in antibiotic consumption rates. Reductions in consumption were observed across all antibiotic classes, including penicillin and β-lactamase inhibitor combinations, macrolides, fluoroquinolones, cephalosporins, and carbapenems.9 Research and Development is now widely supported for new and alternative antimicrobials, new point-of-care diagnostic methods and vaccines, which can all help reduce the reliance on antibiotics.
Finally and probably most importantly, the need for education at all levels of personnel is essential to ensure compliance and full understanding of the problem and solutions. Continued surveillance and rapid reporting mechanisms will help alert us to impending issues, and any further change can be noticed immediately.
All these initiatives are underpinned by the government, and on 14th October 2024, the G7 Health Ministers reported that they would support funding for push incentives, including contributing to existing global pooled efforts to accelerate R&D of novel antimicrobials, vaccines and diagnostics and 20 alternative therapeutics.
References
- Murray, Christopher J L et al. 2019 Global burden of bacterial antimicrobial resistance in 2019: a systematic analysis The Lancet,399, 629 – 655.
- Prioritisation of pathogens to guide discovery, research and development of new antibiotics for drug-resistant bacterial infections, including tuberculosis. Geneva: World Health Organization; 2017(WHO/EMP/IAU/2017.12). ( Licence: CC BY-NC-SA 3.0 IGO.
- Bertagnolio, S., Dobreva, Z., Centner, C.M., Olaru, I.D., Donà, D., Burzo, S., Huttner, B.D., Chaillon, A., Gebreselassie, N., Wi, T. and Hasso-Agopsowicz, M., 2024. WHO global research priorities for antimicrobial resistance in human health. The Lancet Microbe
- Antibacterial agents in clinical and preclinical development: an overview and analysis. Geneva: World Health Organization; 2021. Licence: CC BY-NC-SA 3.0 IGO.
- UK 20-year vision for antimicrobial resistance – GOV.UK
- Confronting antimicrobial resistance 2024 to 2029
- Antibiotic Guardian – Pledge to be an Antibiotic Guardian
- How did singing and dancing pills raise awareness of antibiotic resistance? – UK Health Security Agency.
- Zay Ya K, Win PTN, Bielicki J, Lambiris M, Fink G. Association Between Antimicrobial Stewardship Programs and Antibiotic Use Globally: A Systematic Review and Meta-Analysis. JAMA Netw Open.2023;6(2):e2253806.