Researchers have developed breakthrough technology to commercialise cheaper, safer, aqueous rechargeable batteries.
Led by Dr Oh Si Hyoung of the Energy Storage Research Center at the Korea Institute of Science and Technology (KIST), the team has developed safe aqueous rechargeable batteries to offer a timely substitute that meets the cost and safety needs.
Despite the lower energy density achievable, these batteries have a significant economic advantage as the cost of raw materials is much lower than the more commonly used lithium-ion battery.
The research, ‘Highly safe aqueous rechargeable batteries via electrolyte regeneration using Pd–MnO2 catalytic cycle,’ was published in Energy Storage Materials.
Unpredictable weather has increased the demand for energy storage systems
This summer, the planet suffered from unprecedented heat waves and heavy rainfalls.
Developing renewable energy and expanding associated infrastructure has become an essential survival strategy to ensure the planet’s sustainability in crisis. Still, it has obvious limitations due to the volatility of electricity production.
For this reason, the demand for energy storage systems (ESS) that can store and supply electricity as needed is ever-increasing. However, lithium-ion batteries currently employed in ESS are costly and prone to potential fire, so there is an urgent need to develop cheaper and safer alternatives, such as aqueous rechargeable batteries.
Making aqueous rechargeable batteries safe for commercial use
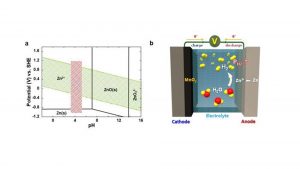
One issue with aqueous rechargeable batteries is that inveterate hydrogen gas generated from parasitic water decomposition causes a gradual rise in internal pressure and eventual depletion of the electrolyte. This poses a sizeable threat to battery safety, making commercialisation difficult.
Until now, researchers have often tried to evade this issue by installing a surface protection layer that minimises the contact area between the metal anode and the electrolyte.
However, the corrosion of the metal anode and accompanying decomposition of water in the electrolyte is inevitable in most cases, and continuous accumulation of hydrogen gas can cause a potential detonation in long-term operation.
To cope with this critical issue, the research team has developed aqueous rechargeable batteries consisting of manganese dioxide and palladium, which are capable of automatically converting hydrogen gas generated inside the cell into water. This ensures both the performance and safety of the cell.
Credit: Korea Institute of Science and Technology
Manganese dioxide does not react with hydrogen gas under normal circumstances, but when a small amount of palladium is added, hydrogen is readily absorbed by the catalysts, being regenerated into water.
In the prototype cell loaded with the newly developed catalysts, the internal pressure of the cell was maintained well below the safety limit, and no electrolyte depletion was observed.
The team managed to overcome critical safety issues
The results of this research effectively solved one of the most concerning safety issues in aqueous rechargeable batteries, making a significant stride towards commercial application to ESS in the future.
Replacing lithium batteries with cheaper and safer aqueous batteries can even trigger a rapid growth of the global market for ESS.
“This technology pertains to a customised safety strategy for aqueous rechargeable batteries, based on the built-in active safety mechanism, through which risk factors are automatically controlled,” explained Dr Oh Si Hyoung.
He concluded: “It can be applied to various industrial facilities where hydrogen gas leakage is one of the major safety concerns to protect public safety.”









