Arthrofibrosis after total joint arthroplasty (TJA) is a complication that can occur after a joint replacement. To prevent arthrofibrosis we need to know more about the complex causes.
Freely mobile joints are classified as ‘diarthroses’ and include all synovial joints of the body, e.g. shoulder, elbow, hip, knee, and ankle, to name the largest. Their structural and functional development during human growth and aging is quite complex and characterised by a variety of cellular actions, reactions, and cell-extracellular matrix interactions. All these joints can develop an arthrofibrosis, a fibrotic joint disease associated with the symptoms of a reduced range of motion (ROM), and possibly pain and swelling, after various forms and degrees of injury, surgery, or infection.
In this short review article, we focus on aseptic (infection-free) arthrofibrosis after total joint arthroplasty (TJA), in particular after total knee arthroplasty (TKA). But according to current data and experience, the symptoms, macroscopic signs, gender differences, pathogenesis, and inflammatory key players apply to all previously-mentioned synovial joints and disease entities.1, 2
The indication for an endoprosthetic treatment of a knee joint is in most cases based on an increasing immobility of the patient due to advanced osteoarthritis (OA) of the affected knee joint.3 The decision for a knee prosthesis by the patient and on the advice of the treating orthopaedic surgeon serves the goal of regaining pain-free mobility.
It is all the more sobering when this goal is not achieved postoperatively during the rehabilitation phase and beyond, despite a correctly inserted prosthesis and otherwise complication-free intra- and postoperative course. Taking into account the very good scientific and socio-economic evidence for the great success of today’s endoprosthetics, the question arises as to the frequency of post-prosthetic arthrofibrosis and their cause.
In this context, it is regrettable that the endoprosthetic registers do not record this complication as a specific complication entity after primary TJA (e.g. TKA), but that arthrofibrosis as such is only recorded when revision surgery appears necessary.4 Sometimes it is still claimed that this complication is a rare one, but recent literature estimates the rates of arthrofibrosis according to, for example, TKA at 0.2% to 10%.5 But the real incidence of the untreated arthrofibrosis is unknown,6 – a circumstance which is extremely unsatisfactory for every patient concerned. If, for example, the knee joint is affected, even a loss of extension of 5° can lead to walking problems and a flexion deficit (flexion less than 90°) can lead to a considerable disability when sitting, climbing stairs, getting in and out of the car, and driving. In addition, these patients suffer from pain, and a loss of mobility and activity at work and in their leisure time, a condition as before the endoprosthetic treatment or possibly even a worse situation.7
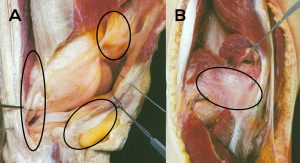
This situation is stressful and unacceptable for the partnership between the patient, the orthopaedic surgeon in private practice, and the treating surgeon. This inevitably leads to the question of the possible causes of post-prosthetic arthrofibrosis. In simple terms, it is a fibrosis and thus a pathological proliferation of connective tissue in the various compartments and joint surrounding and stabilising structures of the capsuloligamentous complex. This is shown exemplarily for the knee joint to illustrate the complexity of the anatomy and the potentially affected regions (see Fig. 1).
Pathogenesis and inflammatory key players of TJA-induced arthrofibrosis
Many scientists still assume that arthrofibrosis, like other organ fibrosis such as in liver or lung, follows a common basic mechanism of inflammation. But Usher et al. take a more differentiated view of this, because the cellular structure and cells’ specialisation of these organs is completely different from that of the joint mucosa, capsule and connective tissue.6 Although all tissues appear to involve a key cellular actor, called a fibroblast, a biological cell type that synthesises extracellular matrix (ECM) such as collagens and produces the structural scaffold (stroma) for animal tissues, it should be noted that lung scaffold fibroblast is not the same as joint mucosa fibroblast, etc.8 Furthermore, other key players are the histiocytes, localised (tissue-specific) cells that do phagocytosis or antigen presentation.
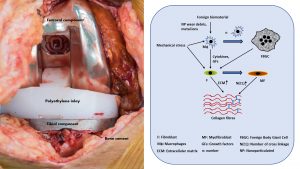
Histiocytes are fully differentiated effector cells of the innate immune system (IS) derived from monocytes/macrophages or Langerhans/dendritic cells.9 These and other cells of the IS such as lymphocytes (adaptive IS) and mast cells, another cell type located in connective tissue and mucosa and important for both innate and adaptive immunity, react to external stimuli such as physical (e.g. mechanical) stress and foreign material/biomaterial (e.g. knee prosthesis, bone cement) and trigger highly complex molecular and cellular reactions, which on the one hand can lead to the formation of a foreign body induced inflammation, and on the other to the formation of myofibroblasts.
Finally, all of the above-mentioned immune cells interact in a highly complex manner with the tissue-specific and function-/task-adapted fibroblasts and influence their local behaviour and molecular expression profile, secretion, connective tissue formation, remodelling and finally scar tissue/adhesions production.6 The contraction of this scar tissue or adhesions lead to the macroscopic and clinical phenomenon of ROM and the underlying immunologically triggered inflammation to pain and swelling (see Fig. 2).
If one considers these correlations, which are only gradually uncovered and understood in all their complexity, the currently still widespread idea that fibroblast is the same as fibroblast appears very naive.
What are the risk factors of developing arthrofibrosis after TJA?
In view of the problem identified, the question arises as to what general and patient-specific risk factors there are. Currently, there is no established method to determine the risk of post-prosthetic arthrofibrosis development. Nevertheless, there are numerous indications of which factors could play a role. The type and extent of existing OA and a too early indication for prosthetic knee joint treatment can be the reason for postoperative athrofibrosis, because surgery is performed into a subchronic inflammation. In addition, previous blunt as well as bone- and joint surface-destructive injuries of the knee joint with conservative and especially surgical treatment can greatly increase the risk.
In order to delay a final prosthetic restoration of the knee, orthopaedic corrective surgery such as repositioning osteotomy with temporary insertion of foreign material (plate and screws) is used in certain cases. This means that ideally, TKA is preceded by two operations, which paves the immunological way for a possible post-prosthetic arthrofibrosis. Other preceded operations could be diagnostic and therapeutic knee arthroscopies with or without foregone injuries (e.g. meniscus or ligamentous tear) that could prime the immune cells located in the synovial membrane of the joint capsule etc. for a later overshooting inflammatory reaction.
Patient-specific factors are inflammatory rheumatic diseases, autoimmune diseases such as Type I diabetes, and the metabolic or age-related Type II diabetes. Other risk factors can be genetic predispositions for mutations in the immune system or genes involved in, for example, collagen metabolism. Finally, but no less importantly, gender differences should also be mentioned. Some studies indicate that women develop arthrofibrosis 2.5-2.8 times more frequently than men, although these studies only refer to arthrofibrosis of the shoulder joint (frozen shoulder) and ACL replacement in the knee joint.10-12 Data on the situation after a primary knee joint prosthesis is currently not available.
Current treatments and future approaches
Among the various therapeutic options currently used and discussed for avoiding or removing post-prosthetic arthrofibrosis, a distinction must be made between non-pharmacological and drug- or diet-based procedures. Non-pharmacological procedures include early physiotherapeutically controlled mobilisation and physical procedures using continuous passive motion (CPM) devices, both immediately after surgery or invasive methods such as surgical lysis and removal of adhesions or manipulation under anaesthesia (MUA), often combined with arthroscopically controlled arthrolysis, after manifested arthrofibrosis.13
Drug treatment is currently essentially limited to the postoperative administration of non-steroidal anti-inflammatory drugs (NSAIDs). Regarding the administration of other anti-inflammatory drugs and so-called biologicals such as antibodies against transforming growth factor-ß (TGF-ß), interleukin-1 (IL-1) or tumour necrosis factor-α (TNF-α), the data and evidence is very confusing and will therefore not be discussed further here.
This also applies to certain dietary forms such as a diet containing omega-3 fatty acids or the intake of so-called food supplements such as antioxidants, but also to exotic ideas such as intra-articular collagenase injections, soft tissue mobilisation techniques, and low dose irradiation prior to surgery.
In our view, the message of this article is that it will be possible to avoid post-prosthetic arthrofibrosis as far as possible. This requires a comprehensive understanding of inflammation and implant immunology, better diagnostic parameters and, based on this, patient-specific safe and evidence-based pharmacological therapies, and non-drug conservative therapies.
References
- Chen AF et al. ‘Arthrofibrosis and large joint scarring’. Connective Tissue Research. 2019;60(1):21-28.
- Krenn V, Morawietz L, Perino G, et al. ‘Revised histopathological consensus classification of joint implant related pathology’. Pathol Res Pract. 2014;210(12):779-786
- Gademan MG, Hofstede SN, Vliet Vlieland TP, Nelissen RG, Marang-van de Mheen PJ. ‘Indication criteria for total hip or knee arthroplasty in osteoarthritis: a state-of-the-science overview’. BMC Musculoskelet Disord. 2016;17(1):463
- Kalson NS et al. ‚International consensus on the definition and classification of fibrosis of the knee joint’. Bone Joint J. 2016;98-B:1479–1488
- Namba RS et al. ‘Risk factors for total knee arthroplasty aseptic revision’. J Arthroplast. 2013;28:122–127
- Usher KM, Zhu S, Mavropalias G, Carrino JA, Zhao J, Xu J. ‘Pathological mechanisms and therapeutic outlooks for arthrofibrosis’. Bone Res. 2019;7:9
- Scholtes SA, Khoo-Summers L, Damico KJ. ‘Presentation and management of arthrofibrosis of the knee: a case report’. Physiother Theory Pract. 2017;33:815–824
- Lynch MD, Watt FM. ‘Fibroblast heterogeneity: implications for human disease’. J Clin Invest. 2018;128(1):26–35
- Gordon S, Plüddemann A. ‘Tissue macrophages: heterogeneity and functions’. BMC Biol. 2017;15:53
- White D, Choi H, Peloquin C, Zhu Y, Zhang Y. ‘Secular trend of adhesive capsulitis’. Arthritis Care Res. 2011;63:1571-1575
- Sanders TL et al. ‘Procedural intervention for arthrofibrosis after ACL reconstruction: trends over two decades’. Knee Surg Sports Traumatol Arthrosc. 2017;25:532–537
- Nwachukwu, BU et al. ‚Arthrofibrosis after anterior cruciate ligament reconstruction in children and adolescents’. J Pediatr Orthop. 2011;31:811–817
- Pujol N, Boisrenoult P, Beaufils P. ‘Post-traumatic knee stiffness: surgical techniques’. Orthop Traumatol Surg Res. 2015;101:179–186
Co-authors
Dr Mustafa Citak (PhD, MD)
Consultant Orthopedic Surgeon, Director of Research Department, Fellowship Director bei Helios ENDO-Klinik Hamburg
+49 40 31970
mustafa.citak@helios-gesundheit.de
www.helios-gesundheit.de/kliniken/hamburg-endo-klinik
www.researchgate.net/lab/Mustafa-Citak-Lab
Dr Jochen Salber (PhD, MD)
Senior Physician Surgery
Head of the Department of
Experimental Surgery
Department of Surgery
University Medical Centre Knappschaftskrankenhaus Bochum –
Hospital of the RUHR-University Bochum
+49 234 32 21819
jochen.salber@rub.de
www.kk-bochum.de
www.researchgate.net/lab/Jochen-Salber-Lab
Please note, this article will also appear in the third edition of our new quarterly publication.