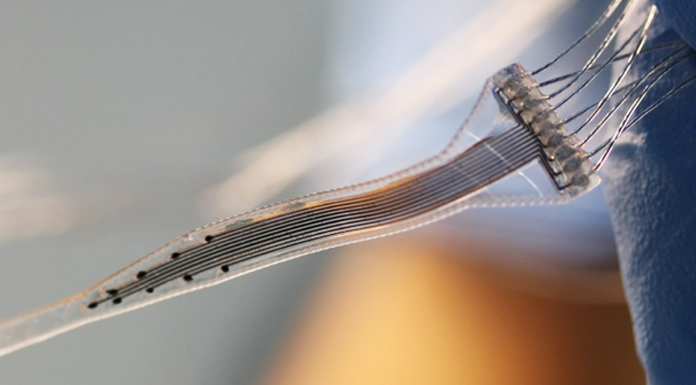
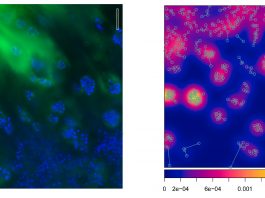
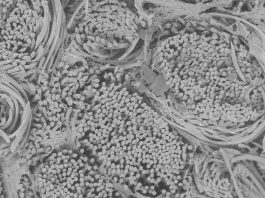
New kind of bioimplants are being developed by Professor Ivan Minev from the Department of Automatic Control and Systems Engineering at Sheffield University.
Bioelectronic implants are widely used in a variety of medical treatments, and yet they are not without their drawbacks. For Professor Ivan Minev from the Department of Automatic Control and Systems Engineering at Sheffield University, UK, there is a clear need to develop new biointerfaces that utilise thermal modulation, chemical signals and mechanical forces alongside electrical signals in order to provide more therapeutic applications in the nervous and cardiovascular systems. Such implantable systems, however, will need to be able to record and stimulate neural activity and integrate seamlessly with soft tissues in the body.
The Innovation Platform’s International Editor, Clifford Holt, spoke to Minev about his research into these new implants and their potential application in areas such as the treatment of epilepsy.
Can you tell me more about your research into a brain implant for treating epilepsy? What would this be designed to achieve? What added benefits would there be over more traditional treatments?
Epilepsy is typically treated with pharmacological interventions (systemic drugs) but with mixed success. As many as a third of cases are resistant to drug treatment and patients are not seizure free. Drugs also produce side effects and there are issues with patient compliance. This motivates the need to develop other lines of treatment.
One option is a bioelectronic implant. There is a precedent here, in that vagus nerve stimulators have been shown to be effective in suppressing seizures. However, they are still associated with side effects and require a continuous stimulation via electrical pulses.
Our idea is to design a brain implant that can detect and terminate seizures at their origin. As a method to terminate seizures, we will use focal brain cooling. This temporarily decreases activity in the affected area and has been shown to stop or shorten seizures. This may also be combined with the delivery of a tiny amount of a drug (e.g. via microfluidics) directly at the seizure origin. These interventions can perhaps be combined to produce a synergistic effect.
The system also requires electrodes, the role of which is to pick up the electrical signals from the brain that indicate the seizure is starting. Such complex implantable systems do not yet exist, but their development can offer an alternative to drug-based treatments.
Why is it important to be able to use more than just electrical signals, which is the mainstay of most other biointerfaces? How do you hope to achieve this?
As previously indicated, combining various modalities of bio-interface can open new opportunities in medicine. Maybe (and this is unknown) they can be combined in a synergistic way; focal cooling combined with focal drug delivery may be much more effective than either of those alone. Combining these with the monitoring of real-time brain responses in the electrical modality can create a closed loop system that can function autonomously.
Another example of multi-modal biointerfaces is microphysiological systems (organ-on-chip). The need here is to emulate the physical environment of the body in vitro. For example, we may wish to subject cells in the petri dish to electrical, chemical, and mechanical stimulation in order to simulate the conditions in the heart. This can then be used in regenerative medicine to produce tissues from stem cells for transplantation.
Despite the fact that these two applications appear quite distant, both are enabled by a bioelectronic interface that ‘speaks the various languages’ of biology (electrical, chemical, thermal, mechanical, and even light).
What does the 3D printing process you have developed entail? What were the main challenges here? And how does it improve on other techniques such as lithography?
From a technology angle, this vision requires the integration of a number of very different materials in miniaturised devices. For example, electrodes require electrically conductive materials and insulating materials, while focal cooling and drug delivery will require microfluidic channels. At the same time, all these building blocks have to be mechanically soft and stretchable to emulate the properties of biological tissues. The materials have to be biocompatible and even promote cell adhesion. Standard microfabrication techniques (such as soft lithography or photolithography) do not offer convenient routes to integrate soft materials in devices. It requires cleanroom infrastructure. Prototyping is a costly and lengthy process.
Multi-material printing promises to resolve this challenge. It has given us the opportunity to develop a ‘lab in a box’. Material deposition and surface conditioning tools (inkjet, extrusion, plasma activation) are combined on one robot that can be programmed to realise different designs. In our recent publications we have described a palette of materials that can be handled by the multi-material printer and used to create biointerface devices, implantable and for in vitro use, and including multi- and single-mode biointerfaces. This gives us freedom to prototype at a fraction of the investment and development costs associated with lithography and cleanroom processing.
Accelerating the time between ideas to prototype will have an impact on how fast these technologies impact medicine (regenerative medicine, prosthetics, drug discovery). There are currently two main limitations, however: the first is the small batch sizes that can be produced using printing; and the other is miniaturisation on par with lithography techniques. This is balanced by the use of materials more suitable for interfacing with living biological systems, multi-modal interfacing, and rapid customisation.
What progress has been made in your development of a device that can control heart cells in culture with both drugs and electrical signals, and how will this feed into the creation of the brain implant?
We have shown that the beating of heart cell cultures can be controlled by a combination of drugs and light, while their electrical activity is monitored by electrodes. We can speed up or slow down their beating activity or lock it to a command frequency. This is a step towards emulating the in vivo environment in a petri dish, where cells receive a mixture of electrical, chemical, and mechanical control commands. In the future, we plan to incorporate mechanical deformation in the printed device so that cultured cells are tricked into believing that they are growing inside a real heart.
The basic principles underlying the two types of biointerface are the same, meaning that a device developed for interfacing heart cells can be adapted for use as a brain implant, or in other places in the nervous system. The position and number of electrodes and microfluidic channels may be different, but both require the exchange of electrical and chemical signals with a machine made of soft tissue-like materials. This fast design adaptation is made possible by our flexible 3D printing process. We have shown this in a recent publication where we produced implants that interface the nervous system at various anatomical levels, from peripheral nerves to the spinal cord and brain in animal models of disease.
You hope to be able to use the brain implant in a personalised medicine approach. How could this be achieved?
We hope to one day be able to match the functions of the implant to the clinical requirements of each patient. This does not only concern adapting the implant’s shape to the specifics of the patient anatomy, however; it also means adapting the therapeutic intervention in real-time. This may be possible with bioelectronic medicine. The example with the epilepsy treatment illustrates this well. The implant will be designed to deploy its action only when a seizure is emerging and adapt the mix of electrical, thermal, and chemical neuromodulation to achieve the best result with minimal temporal and spatial intervention. Much more than just hardware needs to be developed for this. Control and machine learning algorithms will likely be needed to make the device operate autonomously.
Where will your priorities lie moving forwards?
We are focusing on hardware development, especially the part of the system which is in direct contact with neural tissues. One direction for us is to explore what other applications in neuroscience our technology can be useful for. One interesting area is addiction and psychiatric disorders, where brain-machine interfaces may help put the patient back in control of craving episodes.
Another (technological) direction concerns further preclinical validation of implants, their longevity, biointegration, and safety. These are important milestones in the translation of multi-modal soft biointerfaces to the clinic.
Recent articles
- Athanasiadis et al., “Printed elastic membranes for multimodal pacing and recording of human stem-cell-derived cardiomyocytes,” npj Flexible Electronics, vol. 4, no. 1, p. 16, 2020
- Afanasenkau et al., “Rapid prototyping of soft bioelectronic implants for use as neuromuscular interfaces,” Nature Biomedical Engineering, vol. 4, no. 10, pp. 1010-1022, 2020.
Professor Ivan Minev
Professor of Intelligent Healthcare Technologies
Department of Automatic Control and Systems Engineering
Sheffield University
+44 114 222 5614
i.minev@sheffield.ac.uk
Tweet @sheffielduni
www.sheffield.ac.uk/acse/department/people/academic/ivan-minev
Please note, this article also appears in the fifth edition of our quarterly publication.