Professor Melissa A Grunlan and her team, from Texas A&M University’s Department of Biomedical Engineering, have been developing and improving self-fitting SMP scaffolds for craniomaxillofacial (CMF) bone defect treatment.
CMF bone defects arise from trauma, tumour resection, surgical procedures, and congenital conditions. Treatment of CMF bone defects present a unique challenge due to their irregular geometries. Current strategies that rely on biologic and alloplastic grafts are limited by the inability to achieve sufficient bone-to-graft contact. Without good contact, the defect will not heal. Autografting remains the clinical gold standard, but in addition to the issues related to surgical harvesting, bone graft rigidity compromises shaping and ultimately tissue contact, such that graft resorption may occur prior to healing. Synthetic CFM bone graft substitutes that can solidify in situ, such as bone cements, also suffer from poor tissue contact owing to the shrinkage that occurs with solidification (i.e. cure). Moreover, these substitutes often suffer from brittle mechanical properties (leading to post-surgical fracture) and exothermic cures up to 85°C (leading to tissue necrosis). Patient specific, 3D printed PEEK implants have more recently been used to treat CMF defects. However, because of the non-degradable nature of PEEK, the implant is not resorbed by the body and so permanently remains.
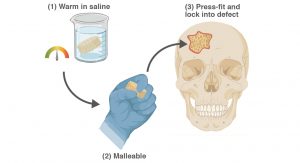
Self-fitting scaffold for bone defect treatment
Regenerative engineering seeks to restore healthy tissues such as bone tissue. A scaffold is a necessary component, serving as a temporary matrix to support cellular processes leading to regeneration. A regenerative engineering approach to treat CMF bone defects is desirable, but requires a resorbable, porous scaffold that could readily achieve conformal fit within irregular defects for good tissue contact. Towards this goal, Dr Melissa A Grunlan and her co-workers have developed ‘self-fitting’ scaffolds based on a biodegradable, shape memory polymers (SMPs). These scaffolds are designed as ‘off-the-shelf’ surgical products, with utility in the absence of exogenous cells or growth factors. The mere exposure to warm saline is all that is needed for the scaffold to soften so that it can be pressed into the defect, with subsequent shape recovery driving its expansion to the defect edges. As the scaffold cools to body temperature, it returns to its hardened or rigid state and so becomes fixed in its new shape within the defect. This process is reversible, allowing multiple manipulations of the scaffold with subsequent exposure to warm saline. Moreover, the scaffold is able to be readily trimmed prior to self-fitting to better match a given defects size and general shape. Thus, the shape memory nature of these scaffolds is expected to produce excellent tissue contact and good healing outcomes.
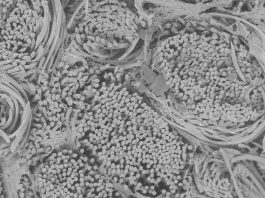
Based on a biodegradable polyester, poly(ε-caprolactone)diacrylate (PCL-DA), porous SMP scaffolds are readily fabricated. A fused salt template is utilised to produce scaffolds with desirable pore sizes as well as pore interconnectivity to permit infiltration of cells and neotissue. PCL is a semi-crystalline polymer and its crystalline lamellae play a key role in its shape memory behaviour. The lamella melts at a certain temperature (Tmelt ~55°C). Thus, upon exposure to warm saline (near the Tmelt), the lamellae begin to melt. This causes the scaffold to become malleable and to also expand within the defect via shape recovery. When the lamellae recrystallise (at body temperature), the scaffold returns to its rigid state, locking in the new shape within the defect.
Improvements
To maximise the potential to heal CMF defects, Grunlan and her co-workers have been working to improve certain properties of the self-fitting SMP scaffolds. These include scaffold rigidity, rate of biodegradation, bioactivity, osteoinductivity, and saline bath temperature. Increasing the rigidity or stiffness of the SMP scaffolds would improve structural support in the early stages of defect healing. Additionally, increasing the rate of degradation would promote integration with adjacent bone tissue as well as regeneration by facilitating neotissue infiltration. Scaffold bioactivity – the ability to induce the formation of the carbonated hydroxyapatite (HAp) mineral on the surface – would promote bonding and integration to adjacent bone tissue. Osteoinductivity would induce bone formation via differentiation of infiltrating cells to a bone-forming lineage. Lastly, reducing the temperature needed to make the scaffold malleable for press-fitting into defects would improve tissue safety, especially when prolonged working times are needed.
The team has studied several SMP scaffold designs to achieve the above properties while maintaining self-fitting behaviour. A polydopamine coating was applied to PCL scaffolds, resulting in bioactivity and osteoinductivity. More recently, co-networks comprised of PCL and polydimethylsiloxane (PDMS) were produced and exhibit bioactivity without the need for a coating. Scaffolds with increased rigidity and accelerated degradation rates were produced with the incorporation of a second polyester, poly(L-lactic acid) (PLLA). Specifically, scaffolds were formed as semi-interpenetrating networks (semi-IPNs) wherein the PCL-DA is crosslinked and the PLLA is not. Finally, the architecture of the PLLA was changed from ‘linear’ to a ‘4-arm, star’, resulting in scaffolds that soften at just ~45°C. Grunlan and her co-workers are currently evaluating the healing potential of these scaffolds in preclinical studies.
References
Melissa A. Grunlan, Dawei Zhang, Cody A. Schoener, William B. Saunders, ‘Shape Memory Polymer Scaffolds for Tissue Defects’. US 9,925,297 B2, issued 3/24/2018
Nail, L.N., Zhang, D., Reinhardt, J.L., Grunlan, M.A. ‘Fabrication of a bioactive, PCL-based ‘self-fitting’ shape memory polymer scaffold’. J. of Visualized Experiments (JOVE), 2015, 104, e52981
Zhang, D., George, O.J., Petersen, K.M., Jimenez-Vergara, A.C., Hahn, M.S. Grunlan, M.A., ‘A bioactive “self-fitting” shape memory polymer (SMP) scaffold with potential to treat craniomaxillofacial (CMF) bone defects’. Acta Biomaterialia, 2014, 10, 4597-4605
Woodard, L.N., Kmetz, K.T., Roth, A. A., Page, V.M., Grunlan, M.A., ‘Porous poly(ε-caprolactone)-poly(L-lactic acid) semi-interpenetrating networks as superior, defect-specific scaffolds with potential for cranial bone defect repair’. Biomacromolecules, 2017, 18, 4075-4083
Please note, this article will also appear in the fifth edition of our quarterly publication.

![1 TEXASAM2 26708[1] bone defect treatment](https://www.innovationnewsnetwork.com/wp-content/uploads/2021/02/1-TEXASAM2-267081-696x392.jpg)