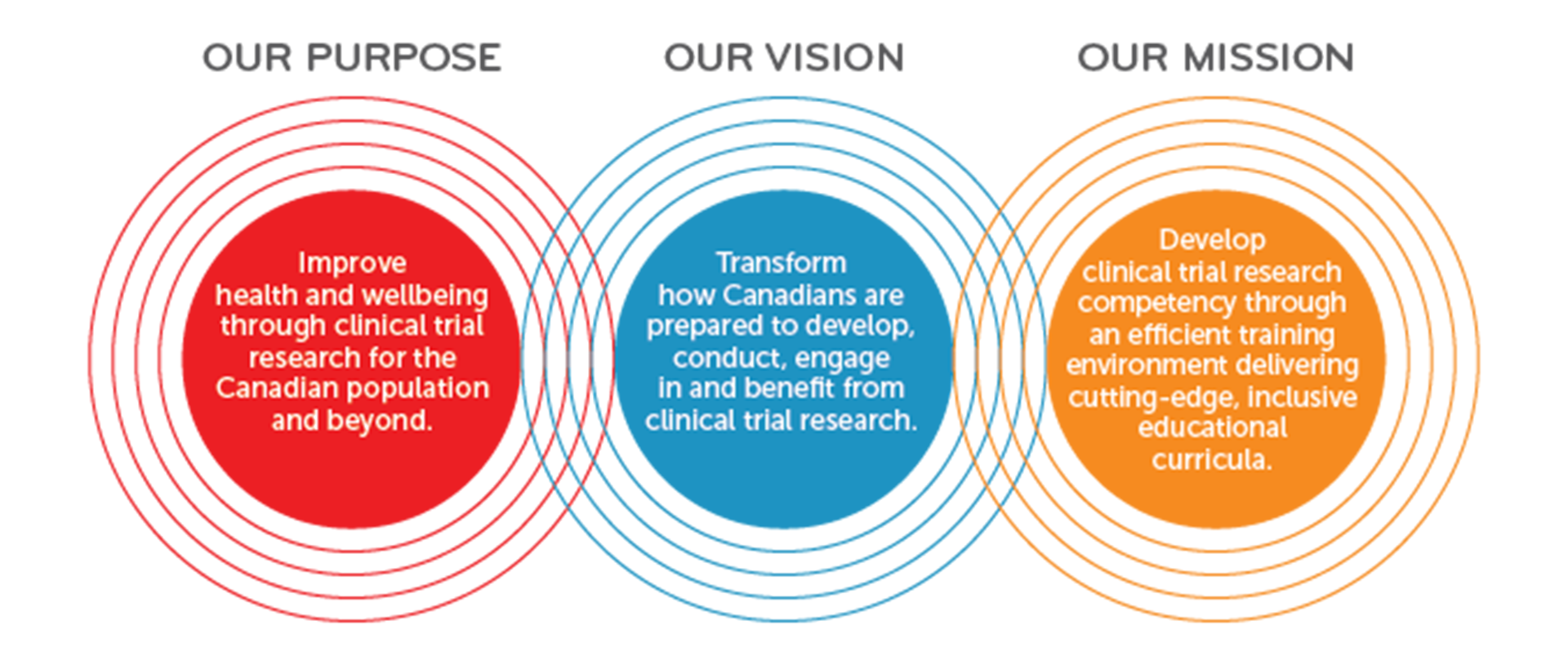
The emergence of a novel Canadian Consortium of Clinical Trial Training Platform, a patient-centred, competency-based e-learning curriculum with mentorship, to support career development.
CANTRAIN is a national programme supported by the Canadian Institutes of Health Research (CIHR) Clinical Trials Fund (CTF), part of Canada’s Biomanufacturing and Life Sciences Strategy led by Innovation, Science and Economic Development (ISED) Canada. CANTRAIN addresses a key aspect of the clinical trial environment — the training of current Graduate Students, Postdoctoral Fellows, and the next generations of Trialists and Clinical Research Professionals in the development and conduct of clinical trials. Through a state-of-the-art Learning Management System (LMS), CANTRAIN provides competency-based courses in clinical research enriched by real-work and real-world experiential activities and expert mentorship.
CANTRAIN curriculum
CANTRAIN is unlike any other training platform. We offer an innovative, competency-based clinical trial training curriculum enriched by experiential learning and expert mentorship. CANTRAIN offers uniquely designed training for four streams of learners, including:
- Graduate and postdoctoral trainees (GPT) – not exclusive to medical and health sciences;
- Clinical trial/research professionals (CTP/CRP) – as assistants, associates, co-ordinators, and managers;
- Clinicians/clinical researchers/trialists, as Principal Investigators (PI); and
- Patients and community partners.

This latest stream aimed at public engagement is further defined to include health charities, policymakers, journalists, social media influencers, and more. Its specific intention is to increase equity, diversity, inclusion, and accessibility (EDIA) for underserved and underrepresented groups interested in working and/or participating in clinical trial research.
The CANTRAIN programmes and platform are available to all Canadian public, academic, and healthcare institutions (hospitals, research centres/institutes) that host Clinical Trial Units (CTUs). Through federal funding, all CANTRAIN programmes are free in Canada. We intend to offer the same portfolio of training programmes on a fee-for-service basis to the private sector (biotech, biopharma, clinical contract research organisations (CCRO), Site Management Organisation (SMO)), to support the training of their own personnel as well as study staff at clinical trials sites across the country. The time will soon come to expand CANTRAIN’s horizons and engage public and private organisations abroad, including in Europe.
CANTRAIN’s innovative approach to clinical trial training is competency-based and scientifically grounded. Such innovation in the adult learning curriculum and in clinical trials research is aligned with the needs of the persona of learners listed above, starting with the Principal Investigator (PI) / Qualified Investigator (QI) responsible for the conduct of clinical trials, assisted by a variety of clinical research professionals (CRP) from co-ordination to management, ethics, and regulatory affairs. We have adopted and are adapting ‘The Levelled Core Competency Framework for the Clinical Research Professional’ by the Joint Task Force for Clinical Trial Competency (JTF) of the Multi-Regional Clinical Trials (MRCT) Center of Brigham and Women’s Hospital and Harvard. This framework includes 47 competencies across eight domains, graded for different levels of expertise and experience. Furthermore, using adult education principles based on cognitive science, CANTRAIN is now innovating by expanding this framework to new groups of learners, namely trialists, patients and community partners.

CANTRAIN is the creation of academic health and clinical researchers from all over Canada who are partnering to collaborate with patients’ engaged organisations, such as Canadian SPOR (Strategy for Patient-Oriented Research). Similar, yet distinct organisations in Europe, such as EUPATI (European Patients Academy on Therapeutic Innovation), and in the US, such as PCORI (Patient-Centered Outcomes Research Institute), are inspiring strategies to engage and embed patient, caregiver and community perspectives and priorities into health research, and therefore speed up clinical trials research outcomes into evidence for better health policy, health services, health experiences and overall health.

CANTRAIN’s strategy focuses on building the knowledge and skills of researchers, patients, and community members to increase the capacity of clinical trials, aiming for knowledge and learner-specific competencies related to clinical trial research.
To encourage Canada’s highly trained students to get involved with clinical trials, CANTRAIN offers three salary award programmes. Supported by the current CIHR grant (2022-2026), and the collaboration with Canadian provincial health research organisations as co-funders, CANTRAIN has been enabled to double the number of awards on offer to master’s and doctoral trainees and postdoctoral fellows. This one-year renewable programme supports these trainees in conducting clinical trial research and following the CANTRAIN curriculum of courses and mentorship.

CANTRAIN’s programming also includes salary and internship awards with practica. There are four dedicated programmes combining courses and mentorship with real-work/real-world experience (RWE) in a clinical trial setting.
- A first programme combines the support of a federal government agency (MITACS) with the private biopharmaceutical sector, ensuring that CANTRAIN can be the rendez-vous portal for graduates and postdoctoral fellows who want to gain experience in industry, the primary clinical trial sponsors. This programme provides trainees with a salary stipendand enables them to gain real-world and real-work experience within a private sector environment for a period of four to 12 months.
- A second programme combines the support of Canadian provincial health research organisations as co-funders to enable CRPs or Early Career Researchers (ECRs) from the public sector to have a combination of virtual and in-person exchanges between their home institution and another public host institution anywhere in Canada.
- A third programme addresses the significant influx of immigrants, many of whom have a medical and/or health sciences background but cannot practice their art and implement their knowledge into their new home. The aim of this internship programme is to enable these skilled immigrants to find a valuable and productive place in their new society based on their initial qualifications. With the retirements of baby boomers and post-COVID human resources changes, there is a need for CRP training and recruitment in both the public and private sectors. These roles range from assistant to associate, co-ordinator, manager, specialists in ethics, informed consent, and regulatory affairs. This programme will allow this group to benefit from CANTRAIN’s training and mentorship programmes along with a pre-determined four to six months practicum in a public or private clinical trial research environment, opening the door to employment.
- A fourth CANTRAIN programme addresses the very specific needs of an essential group, the general public (see above), by giving them the opportunity to spend a day of internship in a public academic Clinical Trials Unit (CTU) with professionals operating various aspects of clinical trials. This approach is designed to develop and strengthen the trust of the population in the conduct of clinical trials sponsored by public grants and private sector sponsors. Back in the community, what better ambassadors than these ‘interns’ to promote participation in clinical trials research, allowing to increase the randomisation of subjects and patients into trials. It also teaches the clinical trial community the importance of having the public and the patients involvement in the whole process of implementing a clinical trial. Acquiring knowledge through education, as CANTRAIN proposes, is mutually beneficial for all parties involved and engaged in the pursuit of successful clinical trials.
Horizon Europe is the world’s largest research and innovation funding programme involving countries around the globe. Canada is part of this most ambitious funding programme as of November 2023. It gives Canadians access to even more opportunities to elevate their research and innovations through global partnerships. Through Horizon Europe, Canada’s talented research and innovation communities, such as CANTRAIN-CTTP, can contribute to the development of solutions to some of the world’s most pressing challenges, mainly toward Horizon Europe Pillar 1 aimed to support excellence in advanced research training, which CANTRAIN provides in the field of clinical trials.

CANTRAIN CTTP includes a level dedicated to ‘Regulatory Compliance.’ At this level, courses are devoted to ICH-GCP (International Council for Harmonisation – Good Clinical Practice), Health Canada, Food and Drug Regulations, and international research ethics standards. Additional courses are being developed to address ITA (Investigational Testing Authorisation) for medical devices, and other jurisdictions such as the US-FDA Chapter 21 of the CFR12 on clinical investigations of drugs, biological products, and medical devices and current Good Manufacturing Practice (cGMP) regulations. The same approach is true for EMA (European Medicines Agency) and UK-MHRA (Medicines and Healthcare products Regulatory Agency). All courses can also be translated to other languages to enable sponsors to qualify sites and conduct trials in different countries.
CANTRAIN CTTP also includes a level dedicated to fundamental (basic or entry level) training, called the Common Core Foundation (CCF). This training consists of 11 courses designed to provide learners of various profiles with a strong knowledge baseline of key concepts in the planning and conduct of clinical trials. Everyone involved in clinical trials should know and understand this information, regardless of their role. Through this level, CANTRAIN modules are tailored, sequenced, and assembled in a way to meet the needs of a wide variety of learners (GPT, CTP/CRP, Trialists and patients/community partners), by selecting optimal content from amongst the domains and competencies of the JTF Core Competency Framework.

CANTRAIN’s vision goes beyond knowledge-based training because it aims at mentoring all people on how to conduct or participate in clinical trials research. It is CANTRAIN’s mission to prepare all learners for the future through an innovative, competency-based clinical trials training curriculum enriched by experiential learning and expert mentorship.
CANADIAN CONSORTIUM OF CLINICAL TRIAL TRAINING – Clinical Trial Training Programmes
Now that Canada has joined Horizon Europe as an associate country under Pillar 1-2-3, CANTRAIN intends to apply with EU partners to form a consortium focusing on providing a multi-lingual, multi-competency, multi-jurisdictional (Health Canada-Regulatory Operations and Enforcement Branch (ROEB), Food and Drug Administration (US-FDA), European Medicines Agencies (EMA), and Medicines and Healthcare products Regulatory Agency (UK-MHRA)) clinical trials training platform across two continents and beyond.
Please note, this article will also appear in the 18th edition of our quarterly publication.





