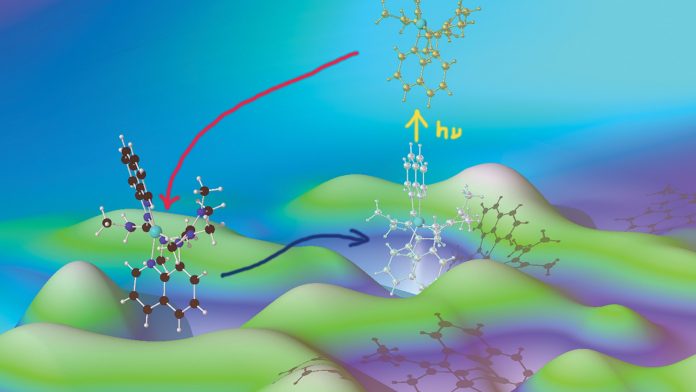
Entatic state model complexes optimise the energies of starting and final configuration to enable fast reaction rates. The Herres-Pawlis group demonstrates that the entatic state principle can be used to tune the photochemistry of copper complexes.
The Herres-Pawlis group is active in bioinorganic topics such as tyrosinase models and entatic state models. Moreover, we investigate the mechanism of robust and fast zinc guanidine complexes in lactide polymerisation and copper complexes for atom transfer radical polymerisation. As the latest result from our lab, we have transferred the entatic state principle into copper photochemistry.
The entatic state principle has been applied for 50 years to interpret thermally activated electron transfer processes at copper centres. Entasis denotes a structural pre-distortion of a transition metal complex towards a reaction transition state, thus facilitating a chemical reaction and, in a narrower sense, enabling faster electron transfer (Fig. 1). This pre-distortion is also discussed as energisation of reactive states and is crucial for efficient catalysis in chemistry and biology. Mostly, the entatic state is observed in copper or iron metalloproteins.
Depending on its oxidation state, the copper atom either prefers a planar configuration, or a tetrahedral arrangement of the neighbouring ligands. However, the binding partner in the protein forces the copper atom to adopt an intermediate arrangement. This highly distorted tetrahedron allows a very rapid shift between the two oxidation states of the copper atom.
Guanidinoquinoline ligands
Biologically relevant, pre-distorted states always involve a metal atom. We examined a model system consisting of a copper complex with specially tailored ligands, so-called ‘guanidinoquinoline ligands’. Recently, we reported a series of bis(chelate), Cu(I) and Cu(II) guanidine-quinoline complex cations [CuII(TMGqu)2]2+ and [CuI(TMGqu)2]+ (see Fig. 2 for overlay of both cations). They display the astonishing feature that their structures are very similar, possessing a co-ordination polyhedron in the middle, between tetrahedral and square-planar environment.
A resonance Raman study of these Cu(I/II) complexes in solution showed that they come into resonance at nearly the same energy around ~3.5 eV by metal-to-ligand charge-transfer (MLCT) and ligand-to-metal charge-transfer (LMCT) processes. We found a dominant Cu-N vibrational mode that couples the optical charge-transfer excitation with the distortion along the reaction co-ordinate, leading from the more tetrahedral Cu(I) to a more flattened – towards square-planar co-ordination – Cu(II) geometry. The donor interplay between guanidine and quinoline units, as well as the large steric encumbrance of guanidines, were found to be crucial for this constrained co-ordination. These model compounds are extremely susceptible to MLCT and LMCT processes, as the predistortion lowers the energy barrier required to enable charge transfer.
In our most recent study, we report on the dynamics of the structural and electronic changes, as induced by an MLCT photoexcitation process, utilising a collection of complementary experimental transient techniques, which provide crucial information on timescales and cover more than four orders of magnitude. With time-resolved optical absorption and emission spectroscopy in the visible and UV range, we identify short-lived electronic intermediate states. Time-resolved IR spectroscopy characterises these intermediates by probing the molecular vibrations in the ligand system. Finally, transient pump-probe X-ray absorption spectroscopy (XAS) focuses on the changes of the Cu oxidation state and its co-ordination sphere in [CuI(TMGqu)2]+, following photoexcitation. The combination of these different experimental tools, with extensive density functional theory (DFT) studies of the excited states and their spectroscopic features, leads to a new comprehensive picture of the reaction dynamics, involving excited singlet and – by intersystem crossing – triplet states.
Pre-distorted states are important for photochemical reactions
Using a wide range of observational methods, as well as theoretical calculations, we showed that the ligands used did indeed put the copper complex into a pre-distorted (entatic) state and were then able to observe the details of the reaction that occurred when light was absorbed. This study was only possible in an interdisciplinary collaboration with physicists, namely Michael Rübhausen from the University of Hamburg and Wolfgang Zinth from Ludwig Maximilian University of Munich, Germany.
The combination of time-dependent UV, infra-red, X-ray and visual fluorescence spectroscopy, allows a detailed picture of the dynamics of the structural changes on a timescale of pico- to nanoseconds. Our studies demonstrate that pre-distorted states are important for photochemical reactions, in other words, for certain biochemical processes which are triggered by light.
The study shows in detail how the process proceeds (Fig. 3):
- From the initial state, (S0, copper in the oxidation state of +1) an electron is transferred from the copper to one of the ligands, by optical excitation into a higher singlet state (S14).
- Within femtoseconds the excited state created decays into another still excited state, known as the S1 state. In this configuration, the geometry is slightly relaxed.
- Shortly afterwards, the system undergoes a spin change. Although one of the electrons has so far remained on the ligand, this electron and its corresponding partner on the copper were spin-coupled. The spin of the electron on the ligand now reverses and this very rapid transition to the so-called ‘triplet state’, within around two picoseconds, removes the spin coupling. This T1 state exists for 120 picoseconds and drops back into the original state again, after reversing its spin once again.
New bioinorganic complexes
All time constants are distinctly shorter, compared with other copper complexes. A complete understanding of all the processes taking place has only become possible through the unique combination of different methods of study.
The detailed analysis of the reaction principle not only improves our understanding of natural processes, it can also help to customise new bioinorganic complexes that imitate Nature, but whose range of reactions extend beyond those of natural molecules. These complexes could also accelerate, or make possible, chemical reactions associated with electron transfers in other areas, too.
The understanding of such fundamental steps will lead to environmentally benign oxidation catalysts, especially in connection with our tyrosinase model complexes which mimic the biological mild hydroxylation activity of tyrosinase. In essence, modern metalloprotein models pave the way to future sustainable catalyst systems.
Reference
B Dicke, A Hoffmann, J Stanek, MS Rampp, B Grimm-Lebsanft, F Biebl, D Rukser, B Maerz, D Göries, M Naumova, M Biednov, G Neuber, A Wetzel, SM Hofmann, P Roedig, A Meents, J Bielecki, J Andreasson, K Beyerlein, HN Chapman, C Bressler, W Zinth, M Rübhausen and S Herres-Pawlis; Transferring the entatic state principle into copper photochemistry; Nature Chemistry, Volume 10, Issue 3, Page(s) 355-362 (2018)
Professor Dr Sonja Herres-Pawlis
RWTH Aachen University
Chair of Bioinorganic Chemistry
Institute of Inorganic Chemistry
+49 241 8093902
http://www.bioac.ac.rwth-aachen.de