A research team from the University of Tsinghua (UT) has revolutionised cathode manufacturing technology to improve solid-state batteries for electric vehicles (EVs).
Why is new cathode manufacturing technology required?
Scientists have been attempting to develop solid-state batteries for EVs because they offer greater energy density and better range than the lithium-ion batteries that are currently in use. However, this aim has remained out of reach, primarily due to issues regarding the composition of the battery’s cathode. Now, UT scientists have developed a novel cathode manufacturing technology in order to overcome this issue.
This study, including a description of the cathode manufacturing process, has recently appeared in Nano Research on 24 March 2022.
Why do scientists want to develop rechargeable solid-state batteries?
Rechargeable solid-state batteries are completely solid, with no liquid components, and have been pursued by scientists as the next generation of energy storage for EV batteries and other climate mitigation applications. This is because they would be lighter, more energy dense, have a faster recharging rate, and offer a greater range than the existing generation of lithium-ion batteries.
The liquid electrolyte utilised in the recharging rate is the medium through which the current flows between the positive and negative electrodes (the cathode and anode, respectively). However, the liquid makes the battery heavy and is also flammable, meaning that fires are not an uncommon occurrence.
Additionally, in a solid-state battery, a solid electrolyte made of ceramic, glass, or a polymer, is much safer because there are no leaks during transit, and it offers improved power density, cyclability, and shelf life.
Can scientists make these batteries work with this new technology?
In order to make solid-state batteries feasible, scientists must design a good cathode that it is capable of a high operating voltage as well as a high area capacity. A high area capacity describes the amount of energy charge in a battery per unit of area for a given period of time. The unit frequently utilised to describe this quantity is the milliampere-hour (mAh)—or the amount of energy charge that will allow one amp of current to flow for an hour — compared to a given amount of area (typically measured in square centimetres, or cm2).
In essence, this measurement, mAh/cm2, indicates how long a battery will last without having to recharge it, for the amount of space it takes up in a device.
“Most of the composite cathode manufacturing technologies that have been explored so far result in batteries that do not even match the performance of existing commercial batteries, let alone exceed them, hitting around 3 mAh/cm2,” explained Jizhang Chen, lead author, from the College of Materials Science and Engineering at Nanjing Forestry University.
Additionally, these cathode technologies also suffer from the requirement of an excessive number of binders and conductive agents. This is to ensure that all of the active particles are uniformly spread out, which reduces the density of the cathode, increases the cost, and produces an excessive amount of resistance at the interface of the cathode and electrode.
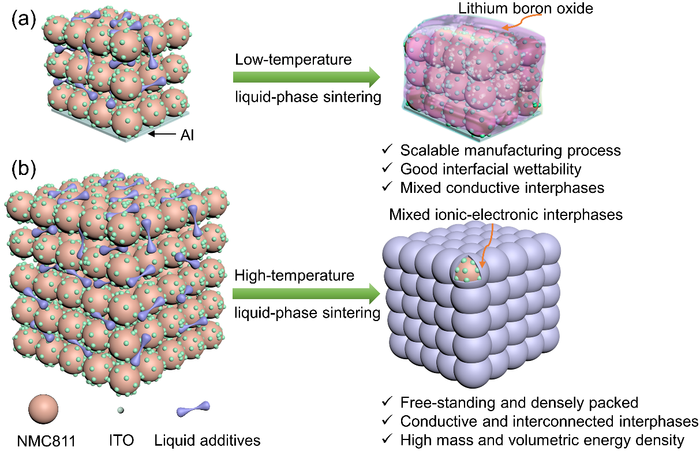
Photo Credit: Nano Research.
How were these issues overcome?
A novel cathode manufacturing technology was developed by researchers in order to overcome these challenges, while offering a high area capacity. The quantity of binders and conductive agents, in this case lithium hydroxide and boric acid, added is substantially reduced (down to about 4% of the overall weight)—these are employed as additives in the sintering process that takes place during cathode formation.
Sintering is a method of compacting a powder into a solid mass, through the application of heat or pressure, without melting it to the point of liquification. In this case however, there remains a liquid phase for at least some components, while others remain as a powder in order to give a boost to the bonding between particles.
Thus, the lithium hydroxide and boric acid, with their low melting points, infiltrate as liquids into a powder of a nickel-rich lithium compound LiNi0.8Mn0.1Co0.1 (NMC811) at a moderately elevated temperature of approximately 350℃. This not only enables intimate physical contact between the powder particles, but it also reduces the need for a high quantity of additives and promotes a densification process.
The resulting composite cathode delivered promises an improved performance, hitting an area capacity of above 8 mAh/cm2 within a wide range of voltages up to 4.4 V. This cathode manufacturing technology is intended to be utilised in manufacturing solid-state batteries with an energy density of 500 watt-hours per kilogram (Wh/kg), easily defeating the 100-265 Wh/kg energy density offered by current contemporary lithium-ion batteries.
To keep up to date with our content, subscribe for updates on our digital publication and newsletter.









