Päivö Kinnunen, Associate Professor at the University of Oulu, discusses the potential of concrete as a major carbon capture and storage solution.
How to design truly environmentally-friendly concrete that is affordable? This multibillion-dollar question is as relevant in business as it is in science and has large implications for the environment, and people. After all, construction is one of the major emitters, with cement manufacturing alone being accountable for 7-8% of anthropogenic greenhouse gas (GHG) emissions. Some scientists think it might even be possible to reverse the emissions by turning cement and concrete into carbon storage to the tune of >1 Gt per year. I tend to think so too, so let me explain why this might be possible and even profitable at some point. But first let us consider the context and introduce some vocabulary that helps us navigate the issue.
We live in interesting times. Society at large has become aware of the fact that the carrying capacities of natural ecosystems have been exceeded on many fronts. While we cannot turn a blind eye to the hard facts about breaching the precarious natural balance, how are we to square that fact with the basic rule of competition, which dictates that overly cumbersome approaches are not viable? That it does not pay to clean the atmosphere? How is it possible to balance the need for short-term economic viability with long-term overall vitality? And not only that, but how to do that under fundamental uncertainty that clouds the evolution of relevant facts and rules of the operating environment?
Turns out that these are fundamental problems that any player in a complex space has to navigate. Lately, complexity science has emerged to deal with these kinds of questions, of how to navigate such complex situations characterised by fundamental uncertainty, where problems are ill-defined and formal risk assessments are literally intractable. Indeed, to commit to formal probability analysis would be too slow, rendering the agent immediately obsolete.
Navigating complexity
Complexity is baked into many of the systems we face. It is also how markets actually function; despite the common illusion, markets do not obey linear prediction curves and well-defined paths, but innovation S-curves (e.g., exponential tech) and unpredictable market disruptions. Change is slow to get started, but if a tipping point is reached it leads to a system-wide ‘phase change’, and the system reaches a new equilibrium. Even though innovation is inherently unpredictable (who knows what will be invented next?), and complex systems defy simplistic causal models, there are ways to successfully navigate complexity. Inherent uncertainty does not necessarily mean high risk.
One potential approach found to be effective is to start with first principles and use them to derive an approach to solve existing problems. Examples of this are Tesla, SpaceX, and the like. When the ideas for those ventures were first introduced, they seemed outrageous to most people. However, the ideas turned out to be extremely competitive and even economically lucrative. In complexity science, it could be said that the reason why they succeeded was that the solutions were well aligned with attractor states in the possibility space.
Complex systems can unfold in multiple trajectories. The trajectories have a strong path dependency, and small changes in the initial state can drastically alter the final outcome. This leads to high unpredictability; so how can one have any confidence in forecasting? Turns out, there may be trajectories that are more ‘fit’ than others, not unlike when a marble is tossed into a vortex simulator at a science museum; we know the marble will spin around the surface, and ultimately make its way down to the bottom because of the state of minimum resistance to the forces of gravity acting upon it. A small change in the initial force can land the marble on the floor instead. Similarly, agents in a complex environment are pulled by one or multiple basins of attraction. So, if we can identify the most critical attractors, and find solutions aligned with most of them, we can have high confidence that it is on the right side of history. In our marble analogy, aligning a solution with multiple attractor states would be the same thing as multiplying the force of gravity acting on the marble.
Closing the loops
So, to return to the initial question: what are the relevant attractor states in the possibility space with regards to sustainable concrete, and what are the first principles that are relevant here? In short, some of the most important would be: accounting for externalities, acting within the available energy bandwidth, and most importantly, closing the loops. I will focus here on the strongest and most important one: closing the loops.
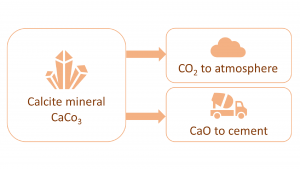
The most relevant attractor in the possibility space of sustainable concrete is arguably ‘closing the loops’. The main issue with current cement manufacturing practices centres around the element of calcium. While calcium is globally abundant, it is not available as pure calcium, but mostly as the calcium carbonate mineral called calcite (CaCO3). That means, when calcium is liberated from the calcite mineral in the cement plant, there will be as much CO2 greenhouse gas generated by weight as there is calcium (i.e. CaCO3(solid) -> CaO(solid) + CO2(gas)). Since CO2 is a gas, for each litre of CaO liberated, the process will liberate about one thousand litres of pure, lethal, carbon dioxide gas to the atmosphere. So, in essence, there is an open loop associated with using calcite feedstock for sourcing calcium. An obvious fix to the issue would be to use another calcium source, such as the wollastonite mineral (CaSiO3), however, due to the large volumes needed (concrete is the most voluminous of all manmade materials), this is not a viable option. There simply are not enough low-cost sources of non-carbonate calcium minerals available.
One family of solutions, which is receiving much-deserved attention currently, is to carbonate cement when it is fresh. This will close the CO2 loop: carbon capture occurs during cement manufacturing, and when the cement is hardened in a special container, it will be injected into the process. The CaCO3 that is formed functions as a binder in concrete, partially replacing calcium silicate that usually fills that role. When this process is applied to recycled concrete, the process can be net-negative in terms of immediate CO2 emissions. Avid readers might notice that the process is not net-negative if the emissions in the initial manufacturing of the original concrete are taken into account. Let us put that aside for a moment. Both of these processes are functional and should be actively pursued, however, they are not fully aligning with long-term attractor states due to their inherent cumbersomeness and scalability issues (carbon capture is not trivial, and the process can only be applied to pre-cast concrete). In other words, while the loops are closed, the approach might not be the most suitable.
The second part of the close-the-loops attractor has to do with recycling. The produced concrete composition shall be such that end-of-life concrete should be recyclable or ‘up-cyclable’. And not only once, but for an indefinite number of cycles. A good example of this recyclability is, for example, the current use of aluminium or steel, which can be melted and purified after use.
Returning to the issue of carbon, and if there are multiple attractor states that one might align with to gain synergy value, interestingly, we have multiple nested loops to consider here. On the one hand, there is the product’s carbon loop, which would suggest that we have to close the loop for each product. But if we zoom out, another picture emerges: the global carbon cycle consists of a fast cycle and a slow cycle. The fast cycle consists of carbon in the biosphere and atmosphere, while the slow cycle consists of carbon moving between rocks, soil, and the ocean, where it takes between 100-200 million years. So, current concrete manufacturing shorts the cycle and pushes carbon from the slow cycle (calcite) to the fast cycle (CO2 in the atmosphere/biosphere). However, it is possible to imagine its counterpart: carbonate minerals synthesised from carbon from the atmosphere (of the biosphere). In my estimation, this can be a much more powerful future attractor state: keep most carbon in the slow cycle. This is of course what the industrial civilisation has powerfully violated for the past hundred or so years, by burning vast amounts of oil, gas, and calcite. Reversing the trend is not easy, even if it was necessary for survival.
Carbon capture and storage
This attractor is, in fact, what is being actively pursued in the field of carbon capture and storage (CCS). Let us call it the CCS attractor. Multiple different approaches exist, but the most relevant to our purposes here is the so-called ex-situ mineralisation of ultramafic rocks. In it, abundantly available magnesium (Mg) silicate (Si) minerals, such as forsterite (Mg2SiO4) or serpentinite (Mg3(Si2O5)(OH)4), are being reacted with CO2 to form a stable carbonate mineral, magnesite (MgCO3). This process has an upside in that it captures CO2 from the atmosphere, but high costs related to mineral processing, carbon capture, mineralisation and finally handling of the generated waste including landfilling. Each ton of carbon captured generates over three tons of mineral waste and costs a fortune (some $150 per ton of CO2, which is more than the price of cement — $100 per ton). This seems like an endless uphill battle.
However, if the carbon capture and storage attractor could be combined with the need for durable and low-cost construction material, a powerful synergy might be gained. Carbon-storing magnesium-based concrete has many advantages. First of all, magnesium is right next to calcium on the periodic table and both are alkali Earth metals, which means that magnesium can do many of the same tricks as calcium. However due to thousands of years of deep dependence on calcium chemistry, we know much more about it than magnesium chemistry. For example, the calcite crystallisation pathway is one of the most studied in science, while the magnesite crystallisation pathway remains enigmatic to this day. What we do know is that magnesium carbonate and magnesium silicate can both be used to make a cement binder. Some magnesium carbonates can hydrate to form an amorphous magnesium (carbonate) hydrate that provides adequate performance to conventional Portland cement, while a magnesium silicate-based binder is analogous to calcium-silicate-based Portland cement. Both systems are built without all the dirty carbon. However, the technology is still not well developed, which is likely due to a lack of insight into the relevant aspects of magnesium chemistry. For example, the main hindrance to using such a magnesium binder, called M-S-H cement, is the slow rate of reaction that does not align well with current fast-paced construction practices. However, a lot of the impressive features of Portland cement concrete stem from the use of highly functionalised chemical additives, so similar additives could be expected to be available for magnesium-based systems.
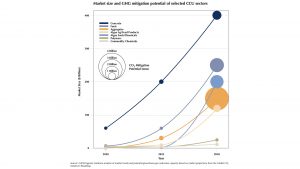
Now we can start aligning with other attractor states to afford an even deeper basin of attraction, and therefore allow for higher returns in the effort invested. In the business of carbon capture and storage, high volume usage ‘waste’ streams are desired. This waste, MgCO3 and silicate, find a suitably sized sink in concrete products. We have concentrated on cement here but, given that cement accounts for less than 10% of concrete, it makes sense to widen the scope. Roughly 80% of concrete consists of carefully sized stones, this is the fraction that carbon capture and storage might first aim to fill. Indeed, the carbon utilisation potential of this fraction is the highest to be found (see graph below).
Another long-term attractor that is gaining strength is what is behind the global sand crisis. Suitably shaped and sized sand is required for making concrete; sand has been used to such a high degree that natural sources are running out. By making carbon-storing sand from abundant minerals, this attractor can be aligned as well. Other related attractors worth mentioning here are the utilisation of mine tailings as Mg feedstocks with simultaneous mining of valuable metals, and utilisation of magnesium from desalination brines. Indeed, these are some of the hot topics that are being seriously considered in many labs, both in academia and in industry.
Wearing the lenses of the current paradigm, it is difficult to perceive what might be possible in the not-so-distant future. The iPhone was seen by the CEOs of Nokia and Blackberry in 2007 as being not very important when it was introduced in 2007. A powerful synergy under the alignment of multiple major attractor states is a force that should not be taken lightly, as it is powerful enough that it can afford a phase change of whole industries. And when that happens, markets are fundamentally disrupted. Ways of making business are forever changed, and the whole deck is reshuffled. Mere knowledge about the possibility can trigger a race to the top between actors in a complex landscape, this time towards more sustainable and durable concrete with which to build a prosperous future.
About the author
Päivö Kinnunen is an Associate Professor at the University of Oulu’s Fibre and Particle Engineering Research unit. He leads a Magnesia-based binders research group (link above) and is the principal investigator in several research projects on the intersection between magnesium-based concretes and carbon capture and utilisation/storage (MAGNEX, CCC, PILCCU, CEMGLASS, and CARBO-CEM). He chairs the RILEM Technical Committee “Magnesia-based binders in concrete”, and leads the circular economy research community (InStreams) in Finland. He sits also on the Materials and Structures journal´s Editorial Advisory Committee.
Please note, this article will also appear in the thirteenth edition of our quarterly publication.