AIMed is a Marie Sklodowska-Curie Innovative Training Network (Horizon2020 MSCA-ITN) training 15 early-stage researchers to develop materials with antimicrobial properties suitable for use on the surfaces of orthopaedic implants to counter the increasing problem of post-operative infection by antibiotic-resistant bacteria.
Current challenges in orthopaedic implant surgery
There are thousands of medical device classes regulated by MHRA and FDA to verify that medical devices approved to be marketed in the United Kingdom and the United States are safe and applicable.
Although these devices save and ameliorate the quality of patients’ lives, one challenge is the potential infection of implanted devices associated with bacterial colonisation and biofilm formation. The biofilm development compromises the immune process at the tissue/implant interface, extends the period of antibiotic therapy, and results in further surgical procedures.
As well as this, bacterial cells in these biofilms can result in a significant increase in antibiotic resistance. Consequently, despite using systemic antibiotic therapy and aseptic surgery procedures, implant infections acquired in hospitals still cause remarkable morbidity and mortality.
What are the key areas of AIMed?
The AIMed network carries out a thorough investigation of the properties of new materials and antimicrobial coatings to ensure that they are feasible for use in future implants. On top of this, the network evaluates antibacterial activity and biocompatibility using appropriate models.
AIMed aims to:
- Develop novel antimicrobial peptides by both classic solid-phase synthesis and recombinant molecular biology methods and create an extensive database with antimicrobial peptide sequences;
- Use novel techniques for immobilising and conjugating antimicrobial peptides on material surfaces to develop smart implants; and
- Evolve nanolayer formations by magnetron sputtering and study laser-induced nano-patterns on metals, ceramics, and polymers.
AIMed is coordinated by the University of Birmingham’s Professor Artemis Stamboulis of the School of Metallurgy and Materials. The project is also associated with the Surgical Reconstruction and Microbiology Centre (SRMRC) in Birmingham, where Professor Stamboulis is leading research into antimicrobial bioinks.
Synthesis and characterisation of antimicrobial peptides
Antimicrobial peptides (AMPs) are known for their broad-spectrum microbicidal activity against antibiotic-resistant bacteria and can even inactivate some viruses. AMPs are produced by the innate immune system of multicellular organisms and constitute a combination of amino acids. They can be incorporated into the implant’s surface coatings, thereby directly offering antimicrobial activity at the implant site.
Professor Artemis Stamboulis leads the Biomaterials Research Group in the School of Metallurgy and Materials to design, synthesise, and characterise novel antimicrobial peptides that mimic the antimicrobial core of human defensins.
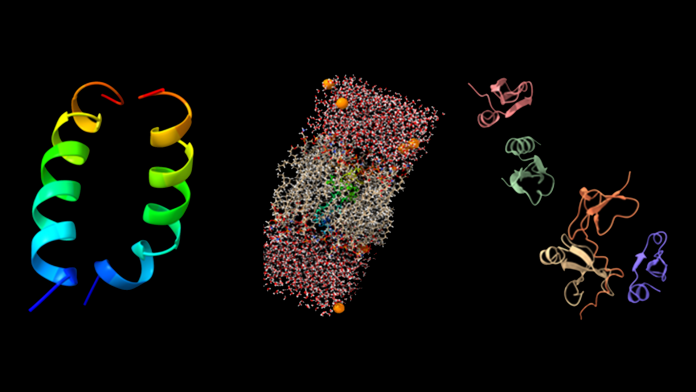
The group’s goal is to computationally design and synthesise AMPs sequences based on human defensins by using solid-phase peptide synthesis. Computer simulations such as molecular dynamics and online tools using Machine Learning algorithms are utilised to predict the structures and biological activities before synthesis (see Fig. 1 above).
Pietro Riccio is an early-stage researcher and synthesises antimicrobial peptides using a CEM Liberty Blue peptide synthesiser. To characterise and purify the peptides, MALDI-TOF Mass Spectrometry, Liquid Chromatography-Mass Spectrometry (LC-MS), High-Performance Liquid Chromatography (HPLC), and Circular Dichroism (CD) are utilised. The synthesised peptides are characterised to determine secondary structures, biological activity against human bacterial pathogens (in collaboration with Dr Sarah Kuehne, School of Dentistry), cytotoxicity, and stability using human cell lines. This work is being conducted in collaboration with Professor Zubair Ahmed of the Institute of Inflammation and Ageing at the Medical School at the University of Birmingham, UK.
The development of human elastin-like polypeptides as a multi-functional and antimicrobial delivery platform is part of this research area performed by the early-stage researcher Laura Colomina Alfaro. This work is supervised by Dr Antonella Bandiera at the Universita Degli Studi di Trieste, Italy. Recombinant DNA technologies are used to produce human elastin-like polypeptides (HELPs) fused with computationally designed peptides based on the sequence of the human β-defensins.
The fusion products that show the desired biochemical and antimicrobial properties against model microorganisms associated with orthopaedic infections are used for prototype hydrogel-like coatings of polymer, ceramic, and metallic surfaces via 3D printing techniques. The expected smart bio-interfaces allow the controlled release of the bioactive domains upon cellular stimuli, like those released during the tissue inflammation process. The development of antibacterial human defensin-like peptides fused to collagen proteins is also carried out in collaboration with Dr Edwige Meurice, Professor Anne Leriche and one of the early-stage researchers, Cristina Cantallops Vilà, at the Université Polytechnique Hauts-de-France, France, as well as Dr Sophie Cazalbou at the Université Paul Sabatier Toulouse, France.
Functionalisation of polymers, ceramics, and metals with antimicrobial peptides
The AMPs can be immobilised onto implant surfaces either chemically through covalent bonding or physically through adsorption and layer-by-layer assembly in Professor Artemis Stamboulis’ laboratory. Effective coating techniques must retain peptide functionalities after they are absorbed onto a coating matrix or tethered to the surfaces of the implants.1
Surface functionalisation of medical metal alloys, polymers, and bio-ceramics through a technique known as plasma nitriding leads to the formation of primary amine groups on the surface used to covalently bond with AMPs. The introduced primary amine (-NH2) functional groups can react with the carboxyl groups of the antimicrobial peptides (AMPs), and consequently, the AMPs can be covalently immobilised on the surface.
For physical adsorption of AMPs, the modification of metallic implant surfaces via mild chemical, heat, and laser treatments are used to optimise the electrostatic binding of AMPs with an added surface binding peptide motif. Different surface characterisations, including X-ray Diffraction (XRD), Atomic Force Microscopy (AFM), X-ray Photoelectron Spectroscopy (XPS), Raman Spectroscopy, and Fourier Transform Infrared Spectroscopy (FTIR), are used to analyse the functionalised surfaces.
Sequentially antimicrobial testing, cytotoxicity assay, and stability assays in physiological conditions are performed to evaluate the successful immobilisation of AMPs on the implant surfaces in collaboration with Dr Sarah Kuehne, Professor Zubair Ahmed, and two early-stage researchers, Mohadeseh Zare and Diana Gomes at the University of Birmingham, UK.
A novel method of sterilisation for orthopaedic applications is being developed by Dr Sophie Cazalbou, Dr Christine Roques, and the early-stage researcher Andrea Marfoglia at the Université Paul Sabatier Toulouse, France. It revolves around the development of a 3D printable hydrogel functionalised with antimicrobial peptides. Andrea Presutti is also an early-stage researcher working under the supervision of Professor Silke Christiansen from Friedrich Alexander Universität Erlangen-Nürnberg, Germany and Professor Stamboulis on antimicrobial bioinks for 3D printing of antimicrobial medical surfaces using different types of polymers such as collagen, PCL, PLA, PLLA-TCP, PLGA-HA, HELP, PEG, and alginate.
In this project, various characterisation techniques are utilised, including 3D cell culturing, immunocytochemistry techniques, rheological assessment techniques, axial compression mechanical testing, adhesive force mechanical testing, Proton Nuclear Magnetic Resonance (H-NMR), Gel Permeation Chromatography (GPC) and FTIR.
A model of exosome-mediated crosstalk between osteoblasts (bone-forming cells) and osteoclasts (bone-resorbing cells) on both 2D cultures and 3D tissue-engineered biomaterials are established by Professor Maria Helena Fernandes and one of the early-stage researchers, Sanjana Vig, at the Universidade of Porto, Portugal. The exosome secretion is comprehensively studied in osteoclast and osteoblast cultures to reveal novel insights into cargo modulation in response to biomaterials.
Laser surface patterning of substrates
Professor Silke Christiansen and Professor Gerd Leuchs at the Friedrich-Alexander-Universität Erlangen-Nürnberg, Innovations-Institut fur Nanotechnologie und korrelative Mikroskopie, Germany, are also cooperating to study Laser-Induced Periodic Surface Structures (LIPSS) as a micromachining technique for implants surface preparation. The researchers hope to produce antimicrobial surfaces and control wettability. The early-stage researcher, Lamborghini Sotelo, investigates the effect of initial surface roughness on laser patterning, chemical etching, and moulding methods of popular implant materials.
Ultra-short laser surface modification of polymers, bioceramics, and metals (Fig. 2) to enhance the biological and antimicrobial properties are being investigated by Dr Albena Daskalova and the two early-stage researchers, Dante Maria Aceti and Emil Filipov at the Institute of Electronics, Bulgaria Academy of Sciences. They are currently studying laser processing in a liquid environment to obtain additional chemical modification and functionalisation of titanium, titanium alloys, and ceramics such as tricalcium phosphate and hydroxyapatite surfaces.
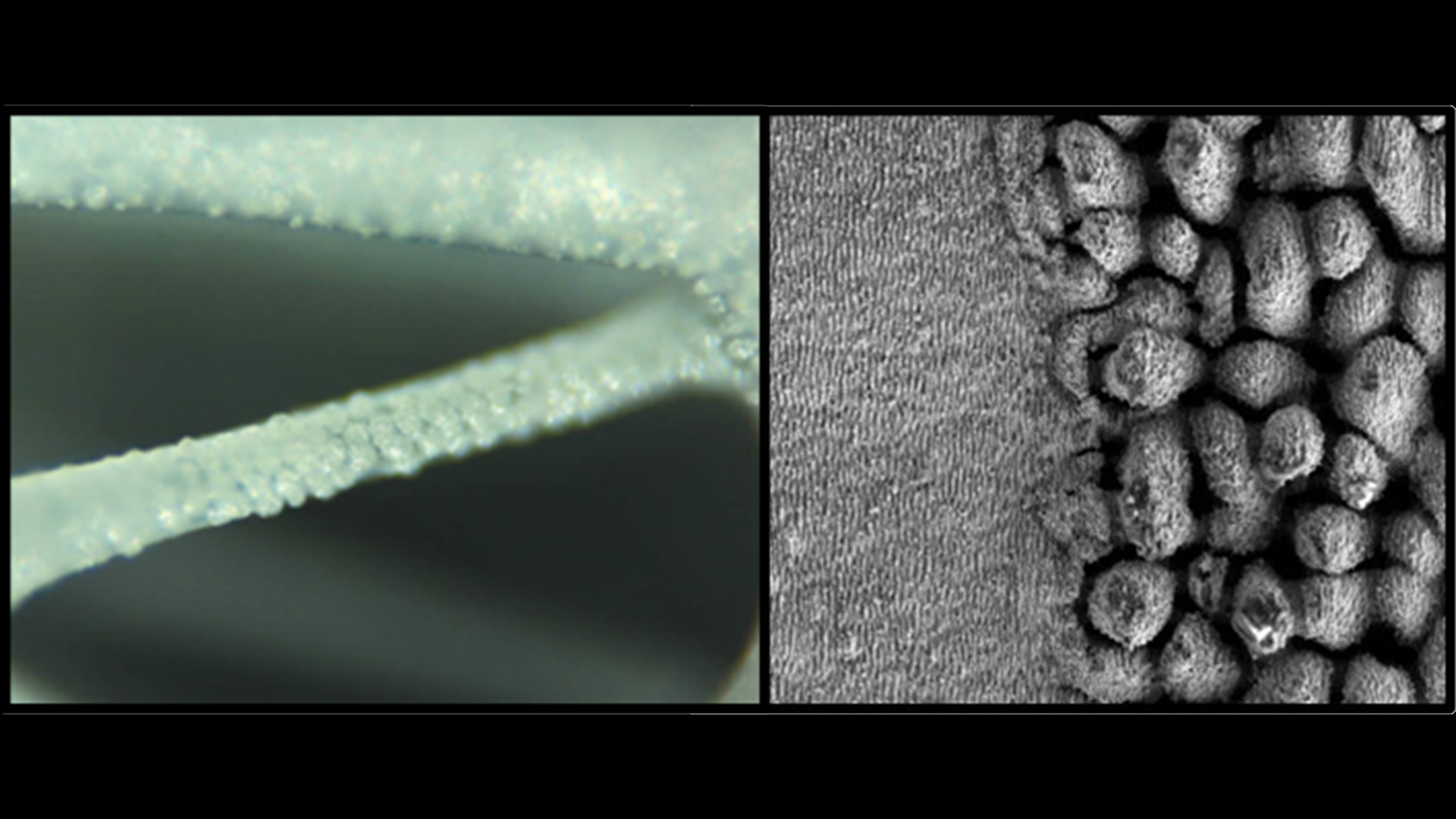
The optimisation of the antimicrobial behaviour of patterned metal and ceramic surfaces using ultra-short pulse laser treatment is led by Drs Wolfgang Schuesselbauer and Hans Amler at Photon Energy GmbH in collaboration with the Friedrich-Alexander-Universität Erlangen-Nürnberg, Germany. In this project, the early-stage researcher Petr Druzhinin is using laser radiation on surfaces to change the nano- and micro-topography, chemical and structural composition and obtain photo-catalytically assisted processes activated by UV laser light. This technique promotes the formation of reactive oxygen species (ROS) that can eliminate all types of bacteria without affecting human health. The researchers successfully developed systems for generating radiation of the second and third harmonics of a picosecond laser with increased resource and stability.2,3,4
Metal-ion substituted calcium phosphate coating for implants
The development of new standards for substituted calcium phosphate-based multi-functional materials and coatings is led by Drs David Grossin, Christophe Drouet, and Fabien Brouillet at the Institut National Polytechnique de Toulouse, France, as part of the AIMed network. Two aspects are considered in parallel to one another: one aspect is optimising different methods to obtain some multilayer apatite precursors with intrinsic antimicrobial properties; the other aspect is the low-temperature coating of titanium alloy substrates and the realisation of scaffolds.
During this project, one of the early-stage researchers, Edoardo Cianflone, utilises various techniques to process and characterise the new materials, including three-fluid nozzle spray drying, a multi-instrumented precipitation reactor, spin coating, focused ion beam-scanning electron microscopy (FIB-SEM), atomic absorption spectroscopy (AAS), and grazing incidence XRD (GI-XRD).
The development of antimicrobial coatings for metals and metal alloys based on metal nanoparticles and organic-inorganic composite coatings is being performed at the Ruđer Bošković Institute, Croatia, by Drs Maja Dutour Sikirić and Jelena Macan, of the University of Zagreb, and the early-stage researcher Ana-Marija Milisav. They produce novel biomimetic multi-layered organic-inorganic coatings with antimicrobial properties and study their stability, build-up, and surface characterisation. The coating with the best properties is selected for further testing as a controlled release system through extensive physicochemical and biological characterisation. For this purpose, different materials such as silver (Ag), copper (Cu), copper oxide (CuO), and zinc oxide (ZnO) nanoparticles are used, and Dynamic and Electrophoretic Light Scattering (DLS, ELS), Quartz-Crystal Microbalance with Dissipation Monitoring (QCM-D), XRD, FTIR, AFM, and TEM are used for the characterisation.5,6,7,8,9
References
1. Innovative Bioceramics in Translational Medicine I: Fundamental Research. Springer Singapore 2022, 159-193.
2. Materials 2021, 7;14(24):7513.
3. Appl. Sci. 2021, 11(6), 2565
4. Polymers 2021, 3;13(15):2577.
5. Nanomaterials 2021, 11(6), 1523.
6. Crystals 2021, 11(7), 792.
7. Colloids Interfaces 2021, 5(3), 38.
8. Soft Matter, 2022, 18, 744-754.
9. Minerals 2022, 12(2), 208.