Dr Dane Walker from Hiden Analytical discusses how differential electrochemical mass spectrometry offers real-time analysis of electrochemical processes.
Electrochemistry is a field of research focussed on chemical change and the relating flow of electrons. British scientist Michael Faraday pioneered and carried out fundamental work in generating electricity from chemical change, creating chemical change using electricity, electrolysis and storing this electrical change in batteries.
Electrochemistry is fundamental in many areas of modern life, with important applications such as batteries, fuel cells, electroplating, electrowinning and hydrogen production.
Until recently, analysis of electrochemical processes was limited to electrical impedance spectroscopy (EIS), linear polarisation resistance (LPR) and remote, post process chemical measurements. Traditional measurements, albeit useful, are limited in their species specificity and measurement speed.
To gain real-time measurements of both dissolved and evolved gases from an electrochemical process, differential electrochemical mass spectrometry (DEMS) was developed. The DEMs technique provides quantifiable insights into cell chemistry by integrating a mass spectrometer system with a customised nanoporous electrode. This allows dissolved species from the electrochemical reaction to be analysed in real time, and these can be correlated to electrode potential and other external parameters such as time. Data gained can be directly correlated to the electrodes’ faradaic current, enabling the determination of key parameters hydrogen evolution and carbon dioxide (CO2) production.
DEMS allows for in-situ mass resolved determination of reaction intermediates, gaseous or volatile electrochemical reactants, and products in real-time. A typical DEMS system comprises an electrochemical half-cell, membrane interface, and a vacuum system that includes a quadrupole mass spectrometer.
DEMS systems are used in many research areas, including:
- Hydrogen oxidation reaction;
- Oxygen reduction reaction;
- CO2 reduction;
- Methanol / formic acid oxidation;
- Ethanol oxidation;
- Ammonia/hydrazine oxidation;
- Cathode effects;
- Battery cycling;
- Electrolyte reactions in batteries;
- Other fuel cells (borohydride, DMFC);
- Mechanistic investigations on methanol oxidation;
- Electrochemical oxidation of organics in (waste-)water treatment; and
- Electrochemical oxidation of CO.
Research from these areas is crucial to many industrial applications such as fuel cell development, hydrogen storage, water treatment & energy storage.
Mass Spectrometry
Mass spectrometry offers a number of advantages over other remote analysis techniques, such as gas chromatography or infrared spectroscopy, such as real-time measurements and wide dynamic range.
A semi-permeable, customised membrane separates the mass spectrometer inlet from the electrochemical cell. Gas and vapour products pass through the membrane to be analysed by the mass spectrometry whilst the electrolyte is held in the cell.
The HPR-40 DEMS system is flexible in its configuration and can be customised to the research that the end-user wishes to carry out. Two electrochemical cells are offered, along with options for direct membrane inlets and micro flow capillaries for headspace analysis.
Real-time electrochemical analysis
Software packages enhanced for DEMS have been developed to allow the user to track evolved species concentrations in real time and with respect to electrode current and voltage. Many third party potentiostats can be interfaced with, and their data can be utilised to synchronise electrode voltage and current with the mass spectral data, allowing the user to easily correlate electrochemical events with species evolution. For example, the electrolysis plot of hydrogen concentration versus potential versus current, over time, provides an insight into electrolysis efficiency which is crucial to electrochemical research.
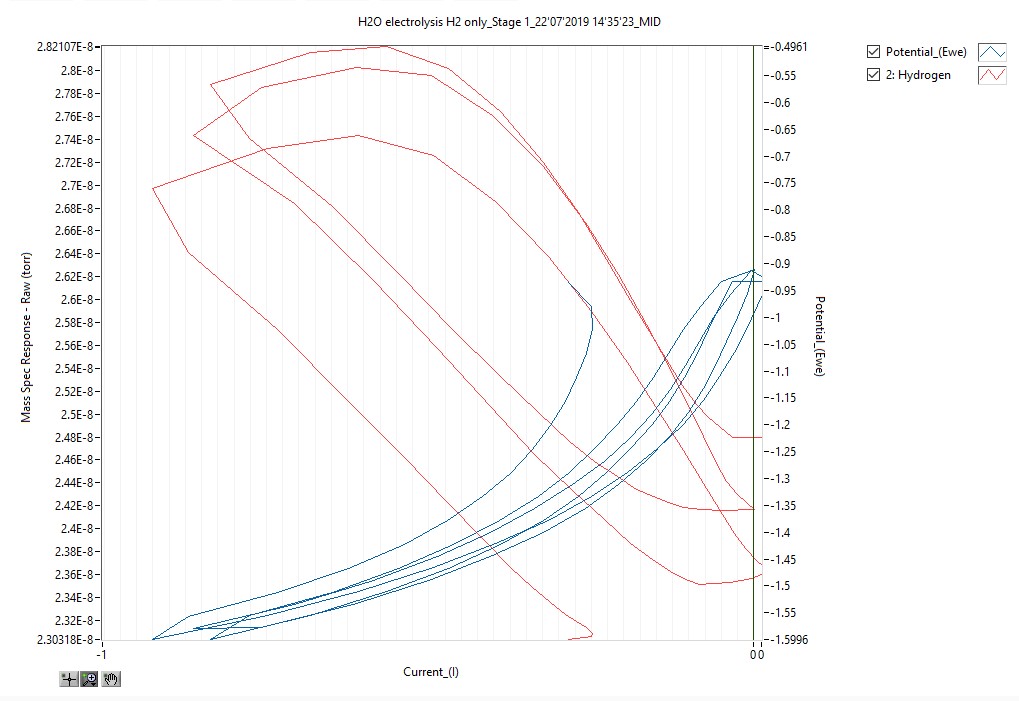
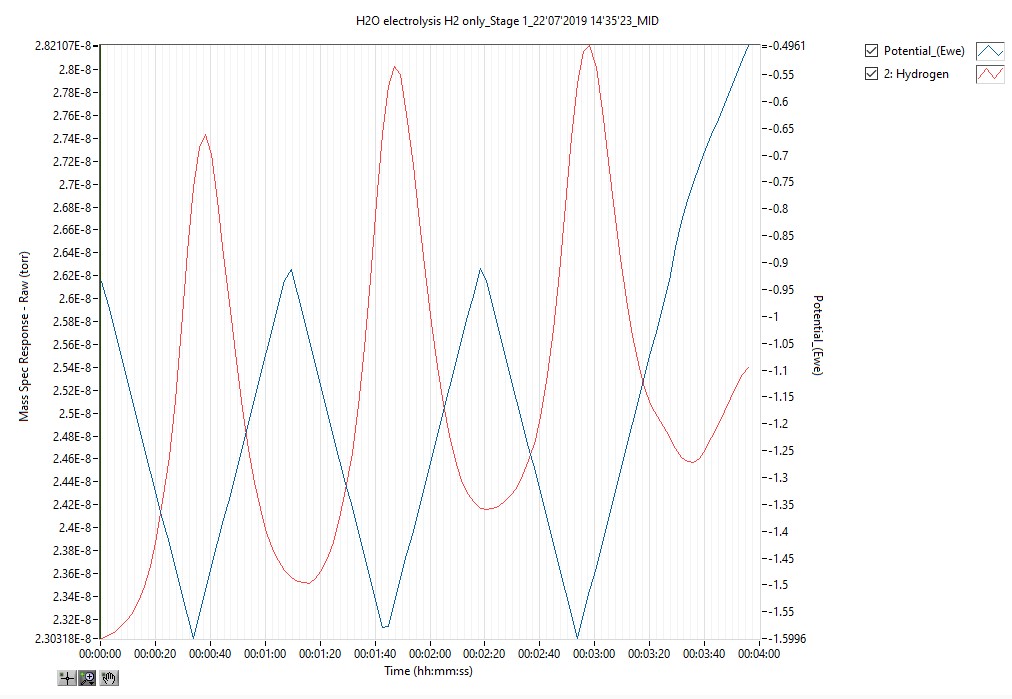
Conclusive advantages of differential electrochemical mass spectrometry
Differential electrochemical mass spectrometry provides real-time analysis of electrochemical processes and is advantageous in a number of areas, including:
- DEMS offers a direct, fast response, real-time analysis solution for all dissolved species and headspace gases;
- A bespoke, customisable system for electrochemical research;
- A wide range of inlets and DEMS cells for broad application range; and
- Application specific software package including interface with external potentiostats.








