Dr Tanja Wintrich and Professor Dr P Martin Sander from the University of Bonn’s Paleontology Department discuss dinosaurs, sea monsters, the evolution of the spine and intervertebral disk and the lessons learned for biomedical research
Dinosaurs and other giant extinct reptiles from deep time are well lodged in the public’s imagination but few realise that their scientific study makes great contributions to understanding evolution, which in turn has profound applications for the medical field. Backboned animals, the Vertebrata, share that fundamental part of their skeleton, the spine, with humans. In fact, the human spine must be understood through its long evolutionary continuity down to the earliest vertebrates that lived 550 million years ago (mya) in the sea. Already at this early time, not only had the spine evolved, but most of its tissue constituents, foremost bone and cartilage, had too.
The spine, of course is segmented, and the segments are defined by each vertebra connected to the one in front and behind it by joints, with the segment boundary running through the middle of these joints. Spinal segmentation is primitive for vertebrates, but the spine as we know it came much later in evolution, with the bony vertebral centrum and neural arch connected by an intervertebral joint and the joints between the zygapophyses. Before this type of spine, there was a continuous rod of cartilage-like tissue, the notochord, which still plays an important role in some fish like the bowfin and the coelacanth. In land animals, the notochord is a transient structure in prenatal development.
Bone originated as an outer cover in the skin of these early, fish-like vertebrates. Only later, in the ancestor of the modern fish and tetrapods (four-legged animals), did bone increasingly become involved in the formation of the inner skeleton of vertebrates, including the spine. This happened about 420 mya. Tetrapods evolved 360 mya, and true land animals appear in the fossil record 310 mya. The key adaptation of these true land animals is found in their complex eggs that regulate gas exchange through membranes and made egg laying on dry land possible. One of these membranes is called the amnion, hence the scientific term ‘Amniota’ for true land animals. For Amniota in particular, the spine became that crucial element in the skeleton, evolving into the structure we know today, that together with the rib cage supports the weight of the body and serves in the attachment of the girdles and limb.
Crucial tissue components in the evolution of the amniote vertebrae and intervertebral joints were bone and cartilage, yet again. In fact, in most bones of the axial skeleton, cartilage is an important precursor developmentally to bone, and it is retained in the joints. In amniotes, evolution resulted in many different spine structures, sometimes repeatedly and at different times. Several such structures are only found in animal groups which are only known from fossils.
Fossil backbones and long necks
It has been pointed out that 99% of all biodiversity is extinct, a plausible statement given the eons of geologic time. But is it not only the countless species that represent this biodiversity, but many larger groups that either vanished without a trace, such as the strange plesiosaurs, or survived in a much-changed form, such as the dinosaurs, which are very much part of our modern world in the guise of birds. Some of the extinction came about gradually and some in short-lived, catastrophic events, known as mass extinctions.
Both plesiosaurs and non-bird dinosaurs evolved extremes in spine morphology and anatomy, far beyond what can be observed in living amniotes. In particular the extremes in the neck are astounding, given that some plesiosaurs had over 70 neck vertebrae, ten times the number of humans and all other mammals, and that in some giant long-necked dinosaurs (the sauropods), a single vertebra was over 1.2m in length! Multiplied by 12 or 15, the resulting necks of these animals represent a challenge to explain to any evolutionary biologist.
Furthermore, the part of the spine supporting the trunk, known as the dorsal vertebral column in amniotes (encompassing the thoracic and lumbar regions of the human spine), is highly variable among different amniote groups. In fact, mammals show the least variation despite their outward appearance of great difference such as between a human and a giraffe. Dinosaurs, flying reptiles, marine reptiles of the Mesozoic, and even the peculiar turtles have an amazingly different spine anatomy in the trunk region, more in accordance with their general dissimilarity in shape and size.
Mammals, in their early evolution during the dawn and reign of the dinosaurs, became very constrained in their developmental pathways and morphospace, yet as our closest relatives they serve as model organisms in the study of evolution and development with an eye on medical applications. However, our greatest knowledge about any single organism is our own species, and what we lack in comparative knowledge about humans is counterbalanced by this unsurpassed in-depth knowledge. These considerations automatically raise the question of what we can learn about spine anatomy in long extinct animals (that are not amenable to the classical dissection and direct observation) for understanding the broader context of the spine in our own species.
What is preserved in fossils: shape, microstructure, soft parts
The vertebrate skeleton has great preservation potential as a fossil because bone, its main constituent, is already highly mineralised when alive. Fresh bone has a content by volume of about 70% bone mineral (hydroxyapatite), the rest is collagen. During fossilisation, the mineral content of bone rises to nearly 100%, replacing the collagen in the process, turning bone to stone. Other tissues involved in spine anatomy fare far less well. Ligaments, tendons, and muscles have very low preservation potential, and the spinal cord has none. Cartilage, on the other hand, is increasingly recognised to survive in fossils, albeit in various states of degradation.
Viewing bone as stone in fossils is actually far from the truth, as was first recognised 180 years ago by early microscopists studying thin slices of fossil bone and seeing a preserved histology in ancient sea monsters such as plesiosaurs and ichthyosaurs. However, only now are the implications of the preservation of osteons, blood vessels, and even bone cells being recognised and questions being asked as to how and why the histology of bone is preserved. To this latter question we do not yet have an answer, but it is at the focus of several projects of our DFG-funded Research Unit 2685 ‘Fossilisation’.
As a spin-off, we started observing fossilised cartilage, commonly associated directly with joints, and we began asking questions about the nature of the intervertebral joints in all those peculiar amniotes populating deep time (and our children’s play). In particular, we were wondering what fossils at the macroscopic and microscopic level would tell us about the evolution of that often-maligned structure in our own spine, the intervertebral disk (IVD).
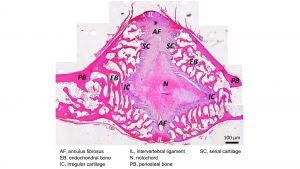
Intervertebral joint shape
A look around the family tree of amniotes, and a visit to the exhibition halls of any major natural history museum, will quickly reveal that the IVD is by no means the ubiquitous type of intervertebral joint despite being the standard for mammals. To appreciate this fact, we need to have a closer look at the structure of the IVD and other types of intervertebral joints. The IVD makes the connection between the two flat articular surfaces of adjacent (above/below, front/back) vertebral centra via a ring of fibrocartilage (annulus fibrosus, AF) surrounding a gelatinous core, the nucleus pulposus (NP). The fibres of the AF are anchored to the bony articular surface (also known as bony endplate), particularly in its periphery. The NP has a different developmental and evolutionary origin, being derived from the primitive vertebrate spine, the notochord. Notochordal cells are large and irregular in shape. The purpose of the NP appears to be cushioning, whereas the AF establishes the connection between the vertebrae, together with the intervertebral ligaments.
Most living reptiles, on the other hand, such as most lizards (including snakes) and crocodiles, have a ball-and-socket joint between each vertebral centrum, consisting of a hemispherical depression into which a hemisphere fits tightly, capping the preceding or succeeding vertebral centrum. The joint surfaces are covered by a thin layer of hyaline cartilage, and the thin space between the joint surfaces is filled with synovial fluid contained by the joint capsule. This type of synovial joint is also seen in mammals, including humans, such as in the hip joint and the shoulder joint, but not in the dorsal spine. The obvious biomechanical advantages of the ball-and-socket joint are its greater mobility and lesser susceptibility to shear forces, a constant threat in IVDs. Accordingly, the giant sauropod dinosaurs of the Jurassic and Cretaceous have massive ball-and-socket joints, sometimes over 50cm in diameter, even in their dorsal spine. Mammals, even giant ones such as mammoths and whales, always have IVDs, although from a biomechanical point of view, one might wonder if we would not be better off with the ball-and-socket construction.
Early Amniota, generally small in stature and weighing in at a few kilogrammes to a few tens of kilogrammes, have a peculiar joint morphology more reminiscent of a fish or a shark than a land animal. The articular surfaces of the vertebrae are deeply sunken in the centre, sometimes with a small hole or canal for the remaining notochord. This condition is termed ‘amphicoelous notochordal’ and is only seen in a few reptiles today, including the ‘living fossil’ Sphenodon, the tuatara from New Zealand. The anatomy of the joint connecting such amphicoelous notochordal vertebrae with a canal has not been studied much, but our work confirmed early descriptions that there is a structure like an AF surrounding the persisting notochord. Certain Mesozoic marine reptiles, in particular ichthyosaurs, also have amphicoelous vertebrae but without a canal for the notochord, a condition unknown in living amniotes.
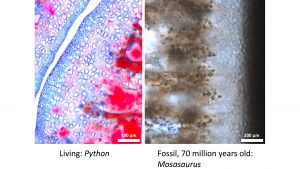
Microstructure of bone and cartilage in fossil intervertebral joints
Centrum and joint surface shape provide only limited information, and the testing of hypotheses of joint type and anatomy in fossil amniotes is only possible through microstructural work, based on the amazingly good preservation of bone, and often cartilage, at the histological level. Our method of choice is known as ‘paleohistology’, the study of thin sections under the polarising light microscope. It only requires a good research microscope and the aforementioned thin sections, slices of fossil bone around 50µm thick produced by careful cutting and grinding of fossil bone. The image visible under the microscope is very much comparable to what is observed in microtome sections of demineralised bone and entire joints and segments of the spine. Staining is not necessary in fossils, as the fossilisation process mysteriously enhances histological features. In most cases, the joint cartilage is preserved as well, albeit in various stages of degradation. Surprisingly, in some cases this even applies to the AF and NP, albeit with a greater margin of uncertainty in the interpretation.
Fibres in connective tissue such as fibrocartilage (of the AF) and the ligamentous joint capsule also leave distinct traces in bone because the anchoring fibers, known as ‘Sharpey’s fibres’, are incorporated by the growing tissue. Different types of a cartilage can also be recognised, in particular the characteristic rows of cells (serial cartilage) making up the cartilaginous joint surface.
Fortunately, the expertise of our lab here at the University of Bonn is in the field of paleohistology, both from the scientific and the technical side. Usually, however, the lab studies the life history of dinosaurs and the aquatic adaptations of ichthyosaurs and plesiosaurs from the histology of their long bones. Reconstructing joints and soft parts from histology was new territory.
Two fossil vertebrae: what was in between?
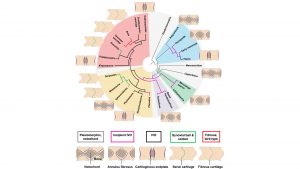
Armed with this knowledge and preliminary discoveries and keeping these considerations in mind, we were wondering if the nature of the intervertebral joint in all major groups of amniotes could be reconstructed and, by mapping it on the family tree, trace its evolution. We thus obtained samples (mostly of fossils) of most major groups of land animals for thin-sectioning, specifically two adjacent dorsal vertebrae preserved in tight articulation. To our surprise, almost all the specimens preserved some kind of cartilage of the intervertebral joint. This was particularly interesting because we found that the orientation of the serial cartilage allowed us to reconstruct the extent of the NP. We found the following five patterns of intervertebral joints in the dorsal vertebrae of amniotes:
- Ancestral amniote joint, in which the notochord is retained, albeit greatly constricted in the middle of the centrum where it has to pass through the notochordal canal. The mechanical connection between the vertebrae is facilitated by an AF inserting into the articular surface of the centrum and intervertebral ligaments inserting in the bone surface of the periphery of the centrum. The vertebral centra of this type are amphicoelous and notochordal. Mobility is limited compared to synovial joints in other parts of the skeleton;
- Incipient IVD combined with amphicoelous, non-notochordal centra. This is an early (and extinct) stage in the evolution of the IVD in which the notochord became separated into segments, in some cases already transforming into an incipient and large NP which must have filled much of the space between the vertebrae. Mobility must have been limited compared to synovial joints;
- Intervertebral disc connecting flat bony endplates. This is the standard IVD. Mobility is limited compared to synovial joints;
- Synovial ball-and-socket joint. The notochordal derivatives, i.e. the NP, are completely reduced, as is the AF. Instead, the vertebrae are physically connected by the joint capsule formed by the intervertebral ligament. Clearly, this joint is the most mobile but also the most resistant to shear forces; and
- Fibrous, bird-type. In this joint type, the articular surface can have different shapes, from flat to saddle-shaped. The vertebrae are connected by fibrous tissue inserting all over the articular surface. The mobility in this joint is very limited.
Tracing evolution: relationships and geological time
The amniote family tree has a deep split into two major branches soon after the appearance of the group on the scene 310 mya. As mammals, we are sitting on one of those branches, called Synapsida. The other branch, known as Sauroposida, includes all living reptiles and birds and a plethora of extinct forms. The best known of these are the dinosaurs (the ones that did not evolve into birds) and the marine reptiles of the Mesozoic, such as plesiosaurs, which went extinct with the dinosaurs 65 mya, and the fish-shaped ichthyosaurs which had already disappeared 90 mya.
In order to understand the evolution of the five joint types, we mapped them on the amniote family tree and then traced evolutionary change using a method called ‘ancestral state reconstruction’ (ASR), also known as ‘character optimisation’. We also put absolute dates on the evolutionary changes based on the fossil record. We found that starting from type one (ancestral amniote joint), evolution proceeded from types one to three, and then on to four or five. However, these transitions took place repeatedly in different lineages and at different times. The classical IVD thus evolved convergently and independently in mammals, in certain marine reptiles (plesiosaurs and relatives) and in early ruling reptiles, which later gave rise to the crocodiles, flying reptiles, and dinosaurs. It seems that the synovial ball-and-socket joint evolved at least three times independently, once in squamate reptiles, once in crocodiles, and once in dinosaurs. Previously, it was believed that crocodiles and squamates inherited their ball-and-socket joint from a common ancestor.
Lessons learned from dinosaurs
The flexibility in the evolution of intervertebral joint types and the repeated evolution of the seemingly most advantageous joint type, the ball-and-socket, raises the question as to why mammals never evolved this type of joint. Pathologies of the IVD are a severe impediment to human health, and one could hypothesise that if primates would have had the ability to evolve a ball-and-socket joint, we would not be faced with he constant threat of slipped disks.
Perspective for evolutionary medicine
Our research on intervertebral joint evolution and that of many other paleontologists on other aspects of anatomy that are found both in dinosaurs and humans as part of the entire amniote family tree offers some basic lessons for evolutionary medicine. Specific insights from our research for the biology of the spine are just emerging, because only now certain questions can be asked. These are of the developmental type, such as the transformation of the embryonic notochord into the NP. This transition may be better understood with our insights into the evolutionary transformation of the notochord to the NP in deep time. Questions are also of the biomechanical type, because by offering different models of IVD and joint functions, biomechanical research on the mammalian spine will be improved. Vice versa, methods developed by biomedical research can be fruitfully applied to fascinating, long-extinct creatures.
However, the most general lesson is that anatomy and physiology are not the sole outcome of an evolutionary optimisation process. Rather, evolution can only optimise locally, being constrained by the history of the organisms and what is left over from mass extinction events. Humans are no exception to this rule.
Dr Tanja Wintrich
Institute of Anatomy
Department of Paleontology
Professor Dr P Martin Sander
Department of Paleontology
University of Bonn
tanja.wintrich@uni-bonn.de
martin.sander@uni-bonn.de
Tweet @UniBonn
www.uni-bonn.de
Please note, this article will also appear in the fourth edition of our new quarterly publication.






