Professor Phillippe Van Cappellen and Stephanie Slowinski from the Ecohydrology Research Group, University of Waterloo, discuss how nutrient legacies affect cultural eutrophication, a process that threatens aquatic ecosystems and water quality worldwide.
The nutrient elements nitrogen (N) and phosphorus (P) are essential to all life forms on Earth. Hence, more N and P would seem like a good thing for the biosphere. However, excessive nutrient availability may actually cause serious harm to aquatic ecosystems, especially rivers, wetlands, lakes, and the ocean’s nearshore zones. Since the second half of the 20th century, human activities, especially intensive agriculture and rising urban wastewater production, have been releasing increasing amounts of N and P into the environment, leading to severe impacts on water quality in many parts of the world. Nutrient enrichment of a surface water body encourages the growth of aquatic algae and macrophytes (aquatic plants), resulting in large quantities of decomposing organic matter, depleting the oxygen vital to other aquatic life, including fish.
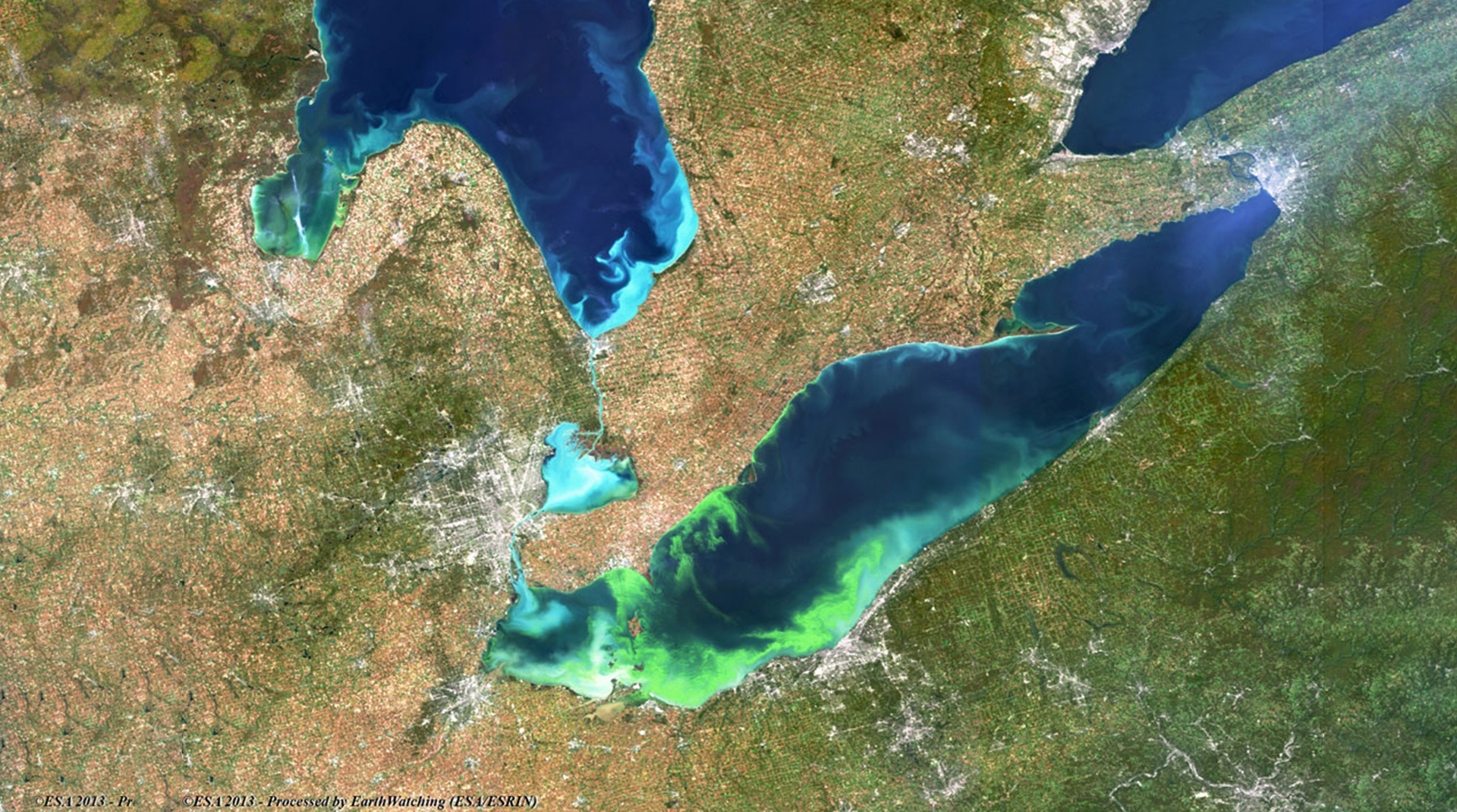
Excessive algal growth also fouls shorelines while certain algal species release toxins that endanger wildlife and humans. These various consequences of human-driven nutrient loading to aquatic environments are known collectively as cultural eutrophication (see Fig. 1).
Mitigating eutrophication
A seemingly straightforward way to combat the negative environmental and health impacts of eutrophication is to reduce the inputs of N and P to a water body. For example, to mitigate the harmful and nuisance algal blooms plaguing Lake Erie, Canada and the United States have agreed to lower the external P loading to the lake by 40% by 2025.1 Similar commitments are being made around the globe. Nutrient loadings associated with discharges from industrial and municipal sources, so-called point sources, are relatively easy to control with existing water treatment technologies. More problematic are agricultural non-point sources. Nonetheless, many beneficial management practices (BMPs) have been developed to control agricultural nutrient emissions. Promising new advances in precision farming may help reduce fertiliser and manure applications further, thus better protecting the environment and saving farmers money.
In many agricultural areas where BMPs have been implemented, the anticipated improvements in water quality have yet to be seen. The culprit is the legacy stores of N and P that have accumulated over time in agricultural landscapes due to the historical applications of fertiliser and manure. That is, the consequences of nutrient pollution are not felt just at the time the pollution occurs, but they can persist for years afterwards due to the delayed release of the legacy nutrients stored in various compartments of the landscape. These compartments include agricultural soils, groundwater, floodplains, wetlands, and reservoirs. Therefore, to tackle the negative impacts of eutrophication, we must not only determine the magnitude of the various nutrient legacy stores in agricultural landscapes and along river corridors but also identify the transport pathways and rates of legacy nutrient transfer to aquatic ecosystems.
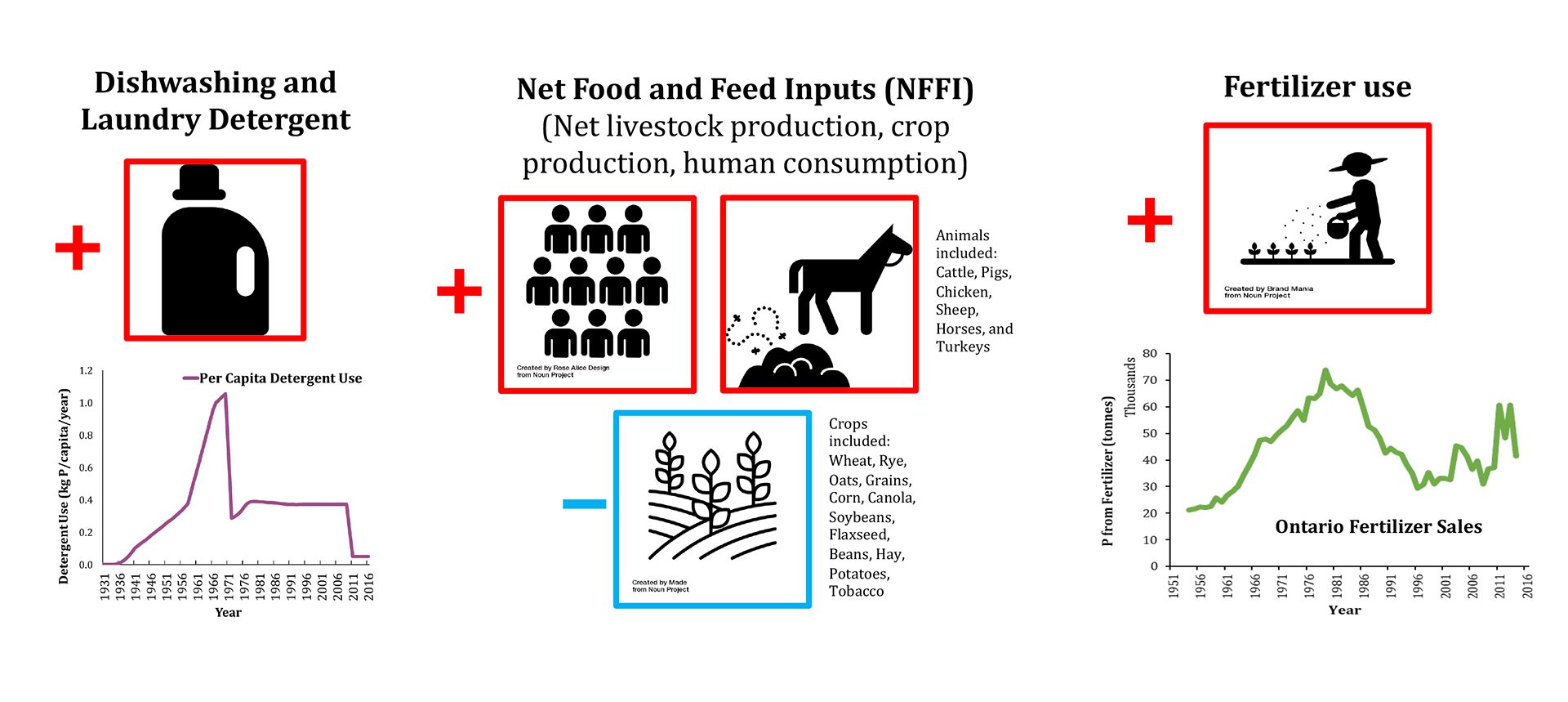
One approach to estimate the magnitude of the legacy of a given nutrient retained in a watershed is to establish the nutrient’s mass balance. In other words, we must calculate how much of the nutrient element is annually imported into and exported from the watershed. If more of the nutrient enters than leaves, then some of it is retained and contributes to the build-up of the legacy. The import estimates are usually based on economic and population (census) data gathered by government agencies and trade organisations. For example, the main P inputs are detergent use, fertiliser applications and net food and feed imports (see Fig. 2). The P export can be estimated from measured time-series data of the P concentration and discharge for the river exiting the watershed. For N, one must also consider the losses to and fixation from the atmosphere of gaseous forms of N, which result from the microbial processes known as denitrification and nitrogen fixation, respectively. When the annual estimates of import and export go far enough back in time, it becomes possible to reconstruct the cumulative (historical) trajectory of the legacy nutrient accumulation in the watershed.2 This can be repeated for all the watersheds in a region of interest.
Influence of the watershed
In the agriculture intensive regions of North America and Europe the vast majority of agricultural nutrient inputs are retained within the local watersheds. For example, for the Grand River watershed in southern Ontario, a study by our colleagues Kim Van Meter and Nandita Basu indicates that over 90% of the cumulative agricultural P inputs since 1900 are still stored in the watershed.3 The Grand River is the largest tributary discharging into the eastern basin of Lake Erie. When all the tributary rivers on the Canadian side of Lake Erie are combined, the amount of P exported by these rivers to the lake between 2003 and 2013 only represents about 12% of the net agricultural P inputs during the same period. Given the strong retention of legacy nutrients, the concern becomes: How permanent are the legacy N and P stores in a watershed? Or, alternatively, how fast are the legacy nutrients being released? To answer these questions, numerical models representing the various legacy stores and release pathways are calibrated using reconstructed nutrient inputs and measured river loads. These models can then be used to predict how legacy nutrients will affect future nutrient export from the watershed.
Legacy P in watersheds is mainly stored in soils underlying farmland, while groundwater in agricultural areas may also accumulate large amounts of legacy N. Model calculations imply that the release of N and P from these environmental compartments may continue to fuel eutrophication in downstream water bodies for many decades, even centuries. For example, if from today on agricultural N use in the Mississippi River basin became 100% efficient, it would still take at least three decades to reach the necessary decrease in N export from the basin to significantly reduce eutrophication in the northern part of the Gulf of Mexico.4 In the study on P legacies in the Grand River watershed mentioned earlier, the recovery time scales could be even longer, on the order of centuries. Overall, the accumulating evidence points to the importance of recognizing the lasting effects of watershed nutrient legacies when implementing BMPs for water quality improvement and assessing their effectiveness. In other words, delays in the recovery of water quality should be expected and should not necessarily be taken as evidence of the failure of the BMPs.
Internal phosphorus loading
In freshwater and nearshore marine water bodies that suffer from eutrophication, much of the imported excess nutrients may end up accumulating in the bottom sediments. Analysing sediment cores can therefore help unravel the past nutrient loadings to the water body and characterise the nature of the sedimentary N and P legacy pools (see Fig. 3).

Like land-based legacies, the release of N and P from bottom sediments can act as a long-term nutrient source that can offset efforts to reduce the external inputs of nutrients from the surrounding watersheds. This is particularly important in freshwater lakes, where P is often the primary limiting nutrient controlling algal growth. Within the sediments, deposited organic and mineral forms of P are broken down, which generates dissolved P that then diffuses back to the overlying water column where it can be used again by algae. This process, where the sediments act as a renewable source of bioavailable P, is known as internal loading. Thus, even when the external P loading to a eutrophic lake or wetland is reduced, the internal loading can continue to sustain high levels of algal growth.
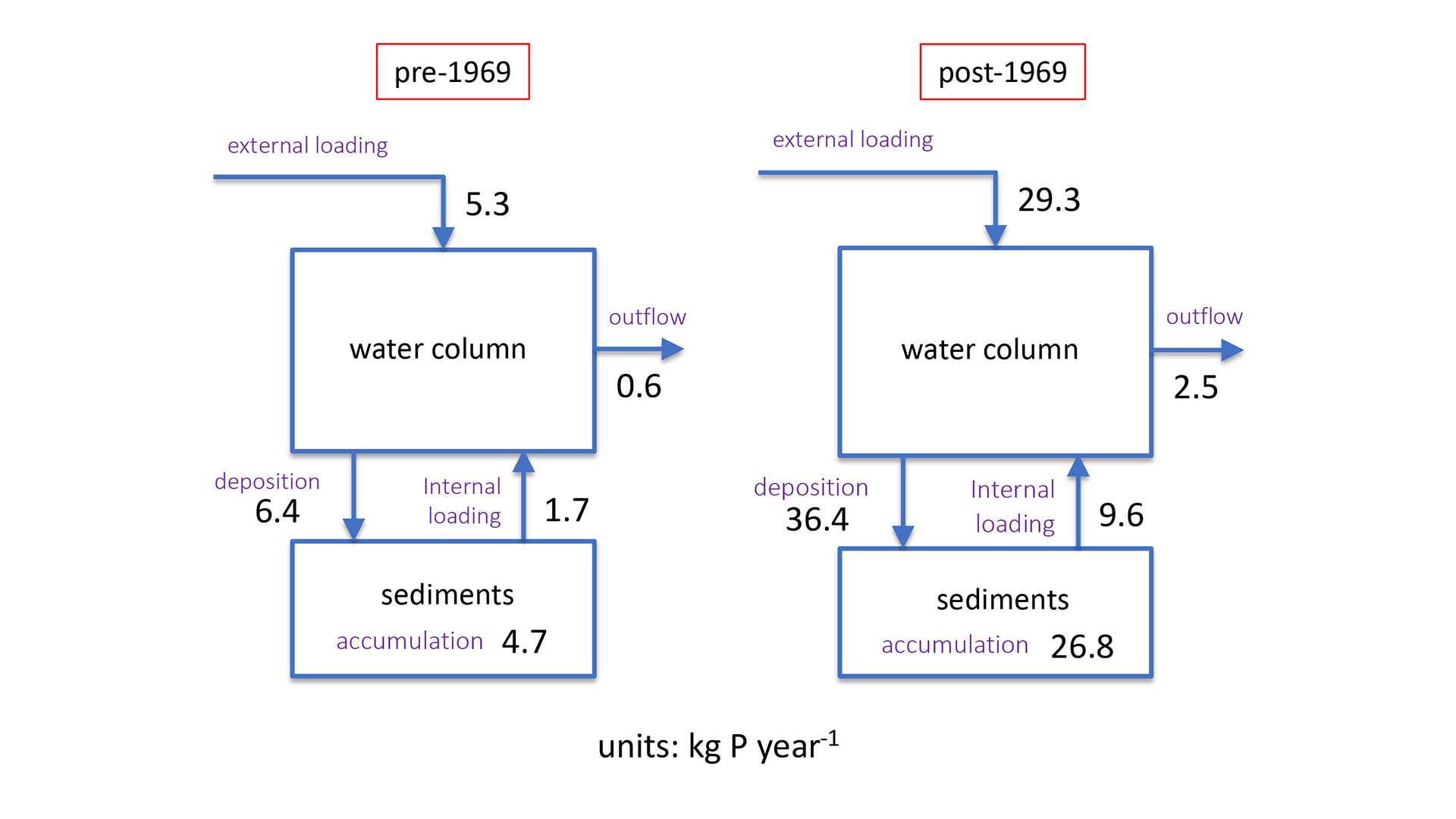
A unique setting to investigate the relationship between internal P loading and eutrophication is Lake 227, which is part of the Experimental Lakes Area in Ontario, Canada. Starting in 1969 and continuing through today, this originally clear water lake has been artificially fertilised by controlled additions of P. This whole-lake manipulation caused the lake to transition from its low-nutrient (oligotrophic) state to eutrophic conditions characterised by recurrent algal blooms. This transition is clearly recorded in sediment cores retrieved from the lake, both in terms of the rate of accumulation and the chemical forms of P.5 Far more P is being stored each year in the post-1969 sediments than before the artificial fertilisation begun. In addition, we found that most of the post-1969 sedimentary P exists in reactive forms, such as phosphate complexes with humic substances.6 Given enough time, these reactive P phases break down and, hence contribute to internal loading to the lake. According to our estimates, should artificial P fertilisation be halted today, the internal loading would continue to supply the algae in Lake 227 with bioavailable P for many decades to come (see Fig. 4).
To further investigate the linkages between sediment P legacies and lake eutrophication, we included the processes controlling internal P loading in a comprehensive model that couples lake hydrodynamics and biogeochemistry.7 This model can simulate the distributions of temperature, dissolved oxygen (O2) and the various forms of P as well as those of other important elements, including carbon, sulphur, iron and aluminium. The model was applied to a eutrophic lake in Norway for which detailed meteorological data plus extensive data on the external P loading, in-lake physical and chemical variables and sediment core analyses were available. On an annual basis, the model-estimated contributions of external and internal P loading are about equal. Furthermore, when the external P loading from the watershed to the lake was turned off in the model, the calculations suggested that remobilisation of legacy P stored in the bottom sediments could sustain the lake’s primary productivity over several centuries. We also used the model to simulate the lake’s response under possible future climate conditions using regional projections for the second half of the century. The model predicts a decrease in ice cover, warmer water temperatures and an increase in internal P loading that, in turn, produces more algal growth. These results are in line with work on other lakes that suggest that ongoing climate warming may be exacerbating eutrophication.
Technological solutions
Several technologies have been introduced to control and reduce internal P loading in eutrophic lakes. Most of them include amending the lake with materials that have a strong affinity for dissolved P. Ferric iron salts are such a class of materials. Ferric iron in water forms oxyhydroxide minerals to which dissolved phosphate ions strongly bind. Hence, by adding ferric iron, dissolved P in the water column is trapped and ultimately deposited at the bottom of the lake, where it remains sequestered in the sediment rather than escaping back to the water column. We simulated such a ferric iron amendment scenario with our model.7 The results are promising, as they show that internal loading can be reduced to nearly half its current value. However, this requires continued amendments of the ferric iron year after year. When the additions cease, the model-predicted algal productivity returns to its pre-treatment level within 15 years. Thus, in-lake amendments should be used in combination with sustained efforts to reduce the external P inputs. In addition, while amendments may be a feasible management intervention for relatively small lakes, they may not be practical or cost-effective for very large water bodies.
Further research
Ongoing work on nutrient legacies in the Ecohydrology Research Group has recently expanded to urban environments. In particular, we are assessing the differences in nutrient export from agricultural versus urban watersheds. We are collaborating closely with municipal and regional partners to evaluate different Low Impact Development (LID) options to reduce nutrient loads to urban streams and wetlands, with a focus on bioretention cells and stormwater management ponds.8 In our work with these engineered systems, we apply many of the lessons we learned about P and N cycling in natural and rural settings, including the importance of accounting for the effects of variable chemical forms of the elements on their fate and transport.9 As in previous studies, we continue to rely on a combination of field sampling, laboratory experiments and numerical modelling to capture the complex interplay of hydrological connectivity, biogeochemical processes, land-use practices, urban nutrient sources and climate drivers. Particular attention is given to the redistribution of nutrients across the urban landscape during storm events. This is motivated by the projected future increases in intensity and frequency of more extreme hydrological events as the climate keeps warming.
In summary, historical nutrient legacies must be taken into account when assessing nutrient enrichment of freshwater and marine ecosystems. While these legacies may create considerable delays in the recovery from cultural eutrophication, we are now in a much better position to incorporate these legacies in predictive models that can inform decision-making and the performance assessment of nutrient abatement measures. Nutrient legacies remind us of Abraham Lincoln’s famous statement: “We cannot escape history”.
References
- Mohamed, M.N., Wellen, C., Parsons, C.T., Taylor, W.D., Arhonditsis, G., Chomicki, K.M., Boyd, D., Weidman, P., Mundle, S.O.C., Van Cappellen, P., Sharpley, A.N. and Haffner, D.G. (2019) Understanding and managing the re-eutrophication of Lake Erie: Knowledge gaps and research priorities. Freshwater Science 38, 675-691, doi:10.1086/705915.
- Van Meter, K.J., Basu, N.B., and Van Cappellen P. (2017) Two centuries of nitrogen dynamics: legacy sources and sinks in the Mississippi and Susquehanna River basins. Global Biogeochemical Cycles 31, 2-23, doi:10.1002/2016GB005498.
- Van Meter, K.J., McLeod, M.M., Liu, J., Thierry Tenkouano, G., Hall, R.I., Van Cappellen, P., and Basu, N.B. (2021) Beyond the mass balance: Watershed phosphorus legacies and the evolution of the current water quality policy challenge. Water Resources Research 57, e2020WR029316, doi: 10.1029/2020WR029316.
- Van Meter K.J., Van Cappellen P., and Basu N. (2018) Legacy nitrogen may prevent achievement of water quality goals in the Gulf of Mexico. Science 360, 427-430, doi: 10.1126/science.aar4462.
- O’Connell, D.W., Ansems, N., Kukkadapu, R.K., Jaisi, D., Orihel, D.M., Cade-Menun, B.J., Hu, Y., Wiklund, J., Hall, R.I., Chessell, H., Behrends, T., and Van Cappellen, P. (2020) Changes in sedimentary phosphorus burial following artificial eutrophication of Lake 227, Experimental Lakes Area, Ontario, Canada. Journal of Geophysical Research: Biogeosciences 125, 5713, doi: 10.1029/2020JG005713.
- Audette, Y., Smith, D.S., Parsons, C., Chen, W., Rezanezhad, F., and Van Cappellen, P. (2019) Phosphorus binding to soil organic matter via ternary complexes with calcium. Chemosphere 260, 127624, doi: 10.1016/j.chemosphere.2020.127624 .
- Markelov, I., Couture, R.-M., Fischer, R., Haande, S., and Van Cappellen, P. (2019) Coupling water column and sediment biogeochemical dynamics: Modeling internal phosphorus loading, climate change responses, and mitigation measures in Lake Vansjø, Norway. Journal of Geophysical Research: Biogeosciences 124, 3847-3866, doi: 10.1029/2019JG005254.
- Ding, B., Rezanezhad, F., Gharedaghloo, B., Van Cappellen, P., and Passeport, E. (2019) Bioretention cells under cold climate conditions: Effects of freezing and thawing on water infiltration, soil structure and nutrient removal. Science of the Total Environment 649, 749-759, doi:10.1016/j.scitotenv.2018.08.366.
- Parson,s C.T., Rezanezhad, F., O’Connell, D.W., and Van Cappellen P. (2017) Sediment phosphorus speciation and mobility under dynamic redox conditions. Biogeosciences 14, 3585-3602, doi:10.5194/bg-14-3585-2017.








