A team of Swedish researchers have devised an innovative method to produce functional cartilage by 3D bioprinting stem cells.
Today, several hundreds of millions of people worldwide are suffering from Osteoarthritis (OA). There is also an increase in OA incidence as the population ages, and OA is likely to rise further with the global obesity epidemic. 3D bioprinting technology and stem cell therapies are often considered to be the future of medicine. To treat OA, the aim of the EU funded project RESTORE is to generate functional cartilage through 3D bioprinting technology.
A Swedish research team within RESTORE has now achieved a major scientific milestone in the treatment of OA; they have been able to generate functional cartilage tissue by 3D bioprinting chondrocytes into OA lesions. The study was recently published in the scientific journal CARTILAGE (Gatenholm, Lindahl, Brittberg, & Simonsson, 2020). A previous study by the research team, demonstrating the formation of functional cartilage by 3D bioprinting stem cells (iPSCs), was published in Scientific Reports (Nguyen et al., 2017). The stem cells were produced by rejuvenating the patient’s cells into stem cells.
Cartilage is composed of specialised cells called chondrocytes that produce a large amount of collagenous extracellular matrix (ECM) abundant ground substances that contains proteoglycans and elastin fibers. Cartilage is classified into three types: elastic, fibrocartilage, and hyaline cartilage. Elastic, fibrocartilage, and hyaline cartilage differ in the number of proteoglycans and collagen. The function of the collagen is to constrain the proteoglycans.
Articular cartilage is the hyaline cartilage found in the knee joint. Unlike elastic cartilage (ear cartilage), fibrocartilage and hyaline cartilage found in the joints of the body, articular cartilage contains collagen II and the proteoglycan aggrecan (ACAN). The only cells found in articular cartilage are chondrocytes which, unlike mesenchymal stem cells (MSCs), are highly specialised and play a unique role in the development, maintenance, and repair of the ECM. Previous studies have shown that transplanted chondrocytes preferably differentiate into cartilage, while MSCs by default differentiate into bone, which is an unwanted feature but similar to OA progression during which bone is usually formed instead of elastic cartilage.
Autologous chondrocyte implantation (ACI) is a cell-based procedure that has been used for more than 30 years to treat traumatic cartilage lesions (Brittberg et al., 1994). Therefore, to achieve the generation of mature articular cartilage after 3D bioprinting, the researchers used primary chondrocytes leftover from autologous chondrocyte implantation (ACI) knee surgery. Knowledge obtained from previous studies that a large number of cells is required to form a tissue using 3D bioprinting was used to design the experiments. Three-dimensional (3D) bioprinting can combine cells with biomaterials and can be used as a tool to distribute cells and biomaterials in the shape of a cartilage lesion or osteoarthritic (OA) defect.
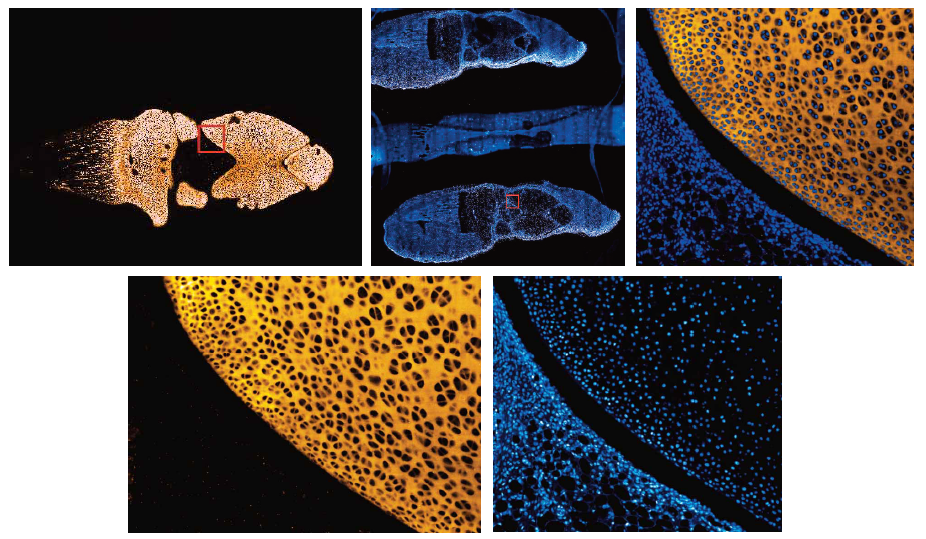
First, an actual (OA) defect was scanned using magnetic resonance imaging (MRI), Computer Tomography (CT), microcomputer tomography (Micro-CT), and a 3D scanner. Out of the devices tested, only the 3D scanner could accurately detect the actual lesion area. The 3D model obtained was then used as a template for 3D bioprinting primary chondrocytes in the shape of a true lesion. The bioprinted chondrocytes formed cartilage tissue fragments and produced collagen IIA isoform type B, analysed by reverse transcription polymerase chain reaction (RT-PCR), earlier proven to be exclusively found in articular cartilage. These levels were higher in the 3D bioprinted chondrocytes than in chondrocytes cultured in 3D micromass, suggesting 3D bioprinting support cartilage formation better than using classic micromass/pellet protocols. The 3D bioprinted chondrocytes also produced ACAN, which is a gene characteristic of articular cartilage.
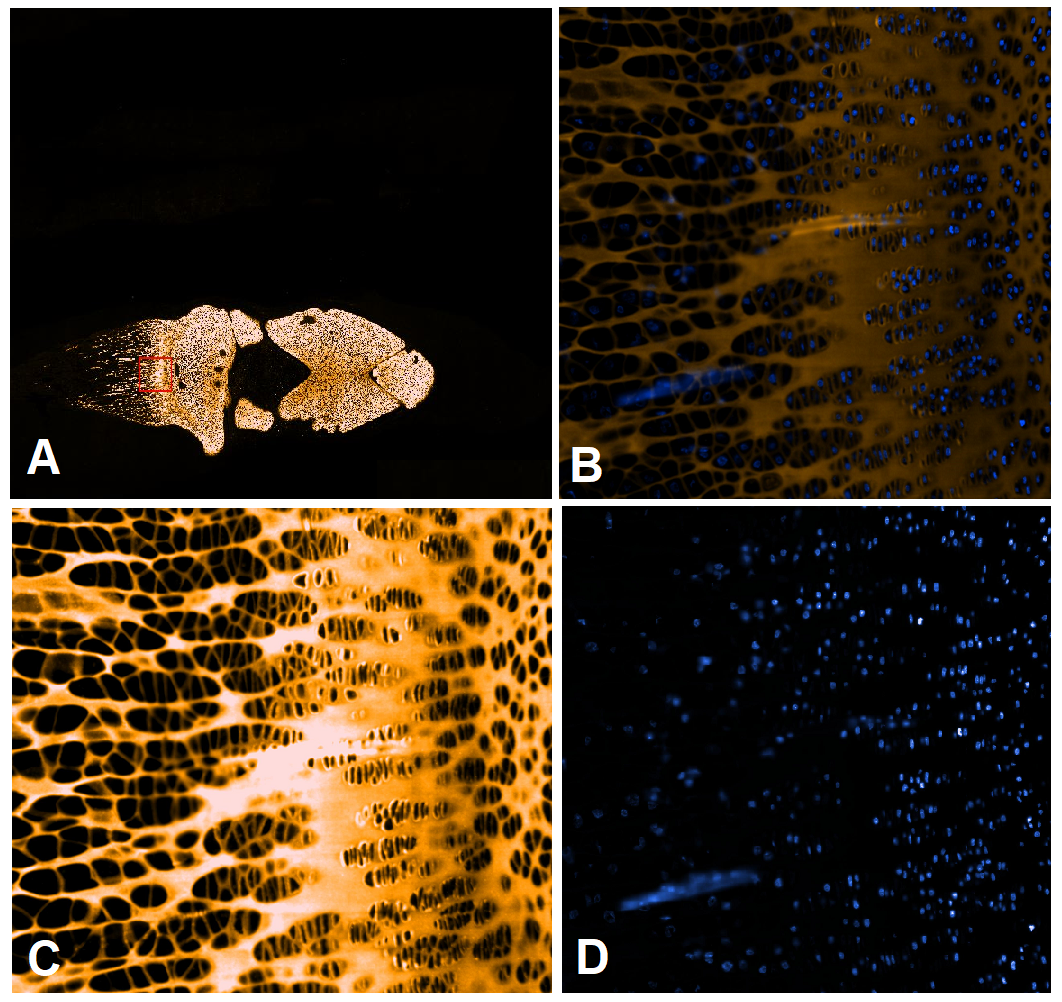
Chondrocytes (the same batch that was used for 3D bioprinting) cultured in monolayer was also used as a control, and RT-PCR revealed that collagen II splice variant type B was undetectable after culturing in monolayer. The scientists showed that the bio-ink for 3D bioprinting supported the chondrocytes to produce mature articular cartilage. This is an important finding and could aid in the design of the next generation scaffolds for articular cartilage injuries or OA when using ink in combination with OA combatting drugs. Such bio-inks based on this, and nano biomaterials are under development in the RESTORE project of the European Union’s Horizon 2020 research and innovation program under grant agreement No 814558. The materials presented and views expressed here are the responsibility of the authors only. The EU Commission takes no responsibility for any use made of the information set out.
The consortium partners of RESTORE project can be found here.
References
- Brittberg, M., Lindahl, A., Nilsson, A., Ohlsson, C., Isaksson, O., & Peterson, L. (1994). Treatment of deep cartilage defects in the knee with autologous chondrocyte transplantation. N Engl J Med, 331(14), 889-895. doi:10.1056/NEJM199410063311401
- Gatenholm, B., Lindahl, C., Brittberg, M., & Simonsson, S. (2020). Collagen 2A Type B Induction after 3D Bioprinting Chondrocytes In Situ into Osteoarthritic Chondral Tibial Lesion. Cartilage, 1947603520903788. doi:10.1177/1947603520903788
- Nguyen, D., Hagg, D. A., Forsman, A., Ekholm, J., Nimkingratana, P., Brantsing, C., Simonsson, S. (2017). Cartilage Tissue Engineering by the 3D Bioprinting of iPS Cells in a Nanocellulose/Alginate Bioink. Sci Rep, 7(1), 658. doi:10.1038/s41598-017-00690-y