Dave Edlund, the Founder and CEO of Element 1 Corp, discusses hydrogen fuel, the issues facing it, and its great potential in fuelling the world’s vehicles.
As momentum for electrifying transportation builds, hydrogen fuel is expected to play a significant role in facilitating the adoption of electric vehicles, vessels, and aircraft.
Given the broad range of power and energy requirements across the spectrum of transportation options, it is not surprising that both fuel-cell-electric vehicles (FCEVs) and battery-electric vehicles (BEVs) show enormous commercial potential. This article will focus on arguments for the high-efficiency conversion of methanol to hydrogen to support FCEV fuelling stations, BEV charging infrastructure, and hydrogen generation onboard FCEVs.
Different needs for different vehicles
It is helpful to think of various modes of transportation in terms of energy consumed between recharging or refuelling events. Energy is expressed in units of kilowatt hours (kWh) or megawatt hours (MWh.) At low kWh, batteries are a convenient and affordable solution for electrifying transportation.
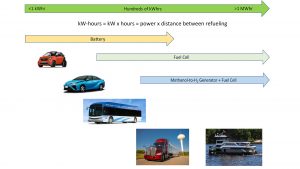
At a few hundred kWh of energy, fuel cells and compressed hydrogen begin to show advantages in range. At MWh of energy, batteries are problematic due to battery weight and because they take a long time to recharge. This segment will likely be dominated by hydrogen fuel cells in a hybrid configuration with a modestly-sized battery bank as an energy buffer. However, storage of MWh of compressed or liquid hydrogen onboard a vehicle or vessel also presents challenges in terms of physical size, weight, and safety concerns. Consequently, liquid hydrogen carriers may be attractive alternatives to large amounts of compressed or liquid hydrogen onboard a vehicle or vessel.
Through 2020, the global growth in FCEVs was steady, but post-pandemic FCEV sales have taken a steep decline. The limited number of hydrogen fuelling stations has constrained the rollout of FCEVs. In the United States, the largest concentration of hydrogen fuelling stations is in metropolitan Los Angeles (Southern California). These stations largely dispense grey hydrogen made from natural gas or water electrolysis using electricity from the regional grid. Hydrogen fuelling stations are expensive to build and may be challenging to site in dense urban environments due to zoning restrictions and safety setbacks resulting from the presence of large quantities of compressed or liquid hydrogen.
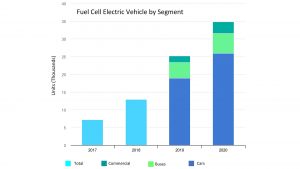
In contrast to FCEVs, BEVs are popular, with all major automobile manufacturers offering at least one model. In the United States, the sale of BEVs has outpaced the sale of FCEVs by more than 40/1, with a forecast of nearly five million BEVs to be sold in the United States in 2030. Given the popularity of BEVs, it makes sense to consider the question of BEV charging infrastructure. Although there are many times more public BEV charging stations than there are hydrogen fuelling stations, the infrastructure build-out for charging stations is still an enormous challenge that is hindered by national electrical grids, which have little room to expand capacity.
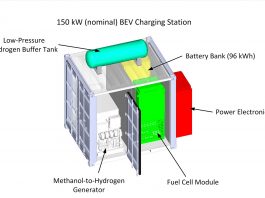
With BEVs, the play for hydrogen is to facilitate grid-independent electrical power generation by converting hydrogen cleanly and efficiently into electricity using a fuel cell. Scaled to hundreds of kW, the hydrogen fuel cell power generator may be either a semi-permanent installation or a portable (truck-mounted or trailer-mounted) solution. It is worthwhile to note that regardless of the source of hydrogen, grid-independent charging stations can be deployed faster and at a lower cost compared to any concepts for rebuilding or expanding national electricity grids, which necessarily comprise generation plants, long-distance transmission, and distribution (from substation to plug).
Producing hydrogen fuel
Most hydrogen today is made by steam-reforming natural gas, although other sources of hydrogen gaining popularity include water electrolysis, ammonia decomposition, and methanol reforming. The choice of feedstock and conversion pathway to hydrogen strongly influences the associated economics ($/kg hydrogen at the point of use) as well as the carbon intensity (kgCO2e/kg hydrogen).
Splitting water into hydrogen and oxygen requires about 50 kWh of electricity per kg of hydrogen produced. Therefore, this process is not feasible for grid-independent power generation. Unlike electrolysis, methanol and ammonia are converted into hydrogen using a thermochemical process, and when considered as a hydrogen carrier each has its unique pros and cons.

As shown in Fig. 3, pure methanol contains more hydrogen in each volume than the same volume of liquid hydrogen, making methanol an attractive hydrogen carrier. Even better, adding supplemental water to methanol increases the hydrogen yield by 50%, and the corresponding volumetric hydrogen content is twice that of liquid hydrogen. Furthermore, methanol is a liquid under all environmental conditions and is readily stored in lightweight, conformal metal or plastic tanks.
Because ammonia is a compressed liquefied gas with a vapour pressure of about 1.5 MPa at 40°C, it is stored in heavy cylindrical tanks. When this geometry is accounted for (poor use of space onboard a vehicle) ammonia is less attractive as a hydrogen carrier compared to methanol. Plus, ammonia is disadvantaged by several unique safety concerns, such that placing large quantities of liquified ammonia in dense urban locations will be challenging at best.
Equally important, the cost of making high-purity hydrogen fuel from methanol/water mix at the point of use (such as a hydrogen fuelling station or BEV charging station) is about $3.45/kg hydrogen assuming methanol is delivered at $500/metric ton. This is attractive and compares favourably to the pump price for gasoline in the United States.
Overcoming issues with hydrogen
Using methanol as a hydrogen carrier has been criticised because methanol contains carbon, and local CO2 emissions are a necessary byproduct of converting methanol into hydrogen. However, as is the case with ammonia and electrical power generation, it is global greenhouse gas (GHG) emissions that are important rather than only local GHG emissions. Just as ammonia synthesis and electricity generation can be associated with low GHG emissions, the same is true for methanol, as shown in Fig. 4.
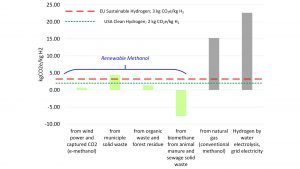
Low and even negative carbon intensities are realised by using renewable methanol. In comparison, high carbon intensity is the result when grid electricity is used to split water into hydrogen and oxygen. Natural gas to hydrogen also yields a high carbon intensity.
Several different pathways to renewable methanol yield the greenest hydrogen, in some cases with a strongly negative carbon intensity due to avoided methane emissions. Renewable methanol results from using biogenic feedstocks (biomass, municipal solid waste, animal manure, and human manure) or carbon dioxide captured directly from air or from industrial waste streams (e-methanol) to make methanol. Renewable methanol has been commercially available for decades, but new demand is spurring a flurry of investment in new capacity. Presently, more than 90 projects globally are announced or underway to produce biomethanol or e-methanol, with a combined anticipated production capacity of eight million tonnes annually by 2027.

The marine sector is poised to be particularly well suited for using methanol as a hydrogen fuel carrier to enable the electrification of vessels. Several current demonstration programs with battery-electric ferries have highlighted the challenge of battery charging these vessels. The M/V Hydrogen One towboat represents the first in a class of working boats that will incorporate methanol-to-hydrogen generators and fuel cell modules onboard to generate 1.4MW of power for propulsion and hotel loads. Onboard bunkering of methanol will give this working boat a range of 550 miles before refuelling. M/V Hydrogen One is scheduled to be launched in 2024.
Please note, this article will also appear in the sixteenth edition of our quarterly publication.