Dr Robert House from the University of Oxford discusses how the identification of O2 formation and its role in voltage hysteresis will inform and direct future material design efforts towards enabling O-redox technology in the next generation of Li-ion batteries.
Almost every waking hour of the day we carry one with us, whether it be strapped to our wrists, planted in our ears, or resting in our pockets. To most of us, they are little more than an inconvenience, failing when we need them most and requiring us to diligently recharge them at the end of each day. But the truth is, we could not lead our modern lifestyles without them. It is, of course, the lithium-ion battery we must thank for powering us through the portable electronics revolution and giving us sleek, slim mobile devices. However, there remains an insatiable demand for longer battery life and ever more compact form factors, which is continuously driving innovation in battery technology.
In addition, there is huge impetus to decarbonise our transport networks in the global effort to mitigate climate change. Up to a quarter of our greenhouse gas emissions come from the transport sector. Road vehicle emissions, such as NOx and particulates, have also contributed to poor air quality and public health in our cities. Electrification offers a very attractive route to tackle these issues by replacing the petrol tank and internal combustion engine with a rechargeable battery and electric motors. Here, the lithium-ion battery is poised to power another electric revolution but, again, the performance demands on battery durability and size are intensely high.
For both electric vehicles and portable electronics, the most critical performance metric of the battery is the energy density (a measure of the amount of energy that can be stored per unit volume, Wh L-1, or per unit mass, Wh kg-1). This is what sets the maximum driving range of the car or how long your phone or laptop can function on a single charge. Following 30 years of advances in cell engineering, the energy density of state-of-the-art Li-ion batteries is now primarily restricted by fundamental limitations of the battery chemistry. New materials are required to achieve the much-needed breakthrough in performance.
The cathode challenge
Inside a lithium-ion battery, the energy is provided by two main functional components called the cathode and the anode. Each of these materials reversibly uptake positively charged lithium-ions (Li+) and an equal number of negatively charged electrons, doing so at different potentials. The total amount of charged species that can be transferred (i.e. capacity, typically measured in mAh, or mAh g-1 normalised per unit mass of material) and the potential difference between these electrodes (i.e. voltage) determines the energy density.
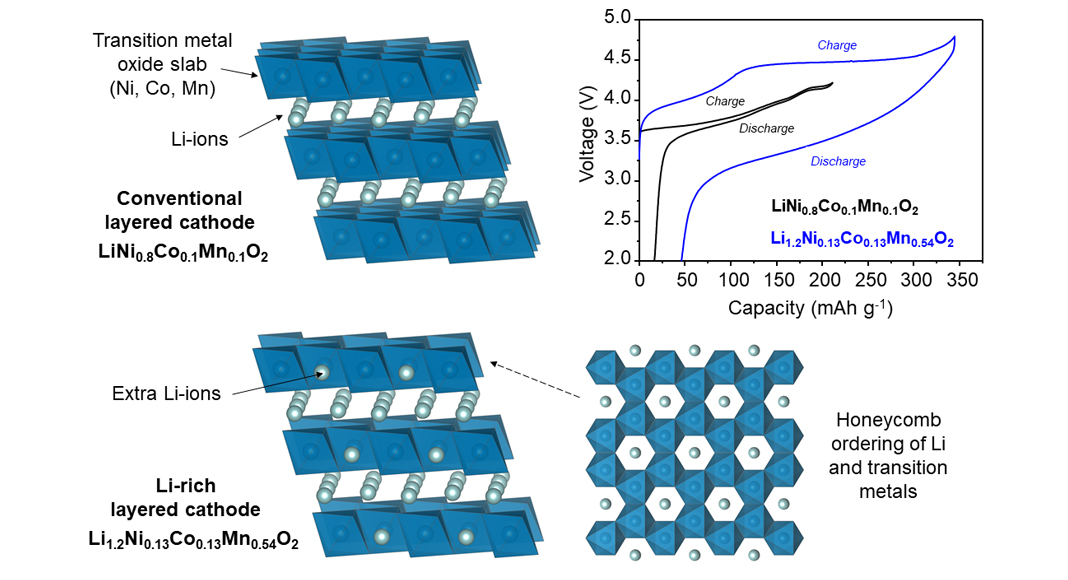
On the anode side, graphite has proven to be an excellent choice of material yielding high capacities (300-500 mAh g-1 with silicon modification) at voltages very close to that of lithium metal (0-0.2 V). Advances are continually being made towards the implementation of lithium-metal, or even anode-less cell designs which will bring another step forward. However, on the cathode side, only incremental improvements upon the original, archetypal material LiCoO2 have been managed so far, leading us towards Ni-rich compounds close to LiNiO2, such as LiNi0.8Co0.1Mn0.1O2, (220 mAh g-1 at 3.8 V). Researchers around the world are intently engaged with finding new cathode materials which surpass these limits as the benefit to overall cell energy density from improvements at the cathode level would be immense.
In order to achieve this goal, close attention must be paid to the composition and atomic structure of the material and a thorough understanding of how it changes when it functions in the battery is essential. On the atomic level, both LiCoO2 and LiNiO2 are formed of alternating layers of lithium ions and transition metal oxide slabs (i.e. CoO2 or NiO2). When the battery is charged, the lithium ions are extracted from the lithium layers and electrons are removed from the Co or Ni ions in a process known as ‘oxidation’ (the reverse is ‘reduction’ and both together give rise to the term ‘redox’). The theoretical limit is set by the number of available lithium ions and of redox-active ions (i.e. transition metals) capable of storing electrons through redox in the material.
The promises and pitfalls of lithium-rich cathodes
To boost this theoretical limit, research attention is heavily focused on so-called ‘lithium-rich’ (or Li-rich) materials where some of the Ni or Co ions within the transition metal oxide slabs are substituted for extra lithium ions (giving Li(LixTM1-x)O2 where the transition metal(s) TM = Ni, Co, Mn, such as Li1.2Ni0.13Co0.13Mn0.54O2) thereby boosting the maximum capacity. However, this modification necessarily reduces the number of transition metal ions available to exchange electrons, raising the question of whether the material is even able to support the lithium extraction.
Strikingly, almost all of the lithium ions can be removed from these lithium-rich compounds at a high voltage that extends beyond the limits of the transition metal redox capacity. In the case of Li1.2Ni0.13Co0.13Mn0.54O2, which is particularly relevant to industry due to low Ni and Co contents, the charging capacity can reach 350 mAh g-1 at an average voltage of 4.2 V which represents an almost two-fold increase in cathode energy density compared with conventional materials. As the battery is discharged, most of this capacity can be recovered. However, there is a pronounced drop in voltage which is referred to as ‘first-cycle voltage hysteresis’. Understanding why this happens is a critical step towards realising these high energy density cathodes in commercial batteries, but it has proven to be a considerable challenge for the research community.
Since the discovery of these compounds, researchers have managed to identify the participation of oxide ions in the redox process (termed oxygen redox or O-redox), offering an explanation for the source of extra electrons. Initially, it was thought that oxidised oxide ions at the surface were reacting with the electrolyte to form CO2 and O2 gas which was irreversibly released from the material yielding the extra capacity. However, it quickly became clear that this mechanism only accounted for a small fraction of the charging capacity and could not explain the reduction process observed on discharge. Instead, it is now established that gas evolution occurs only at the surface and a different, reversible, oxygen redox process in the bulk of the material must be occurring. Nonetheless, the nature of this process has remained elusive.
Uncovering the nature of oxidised oxygen
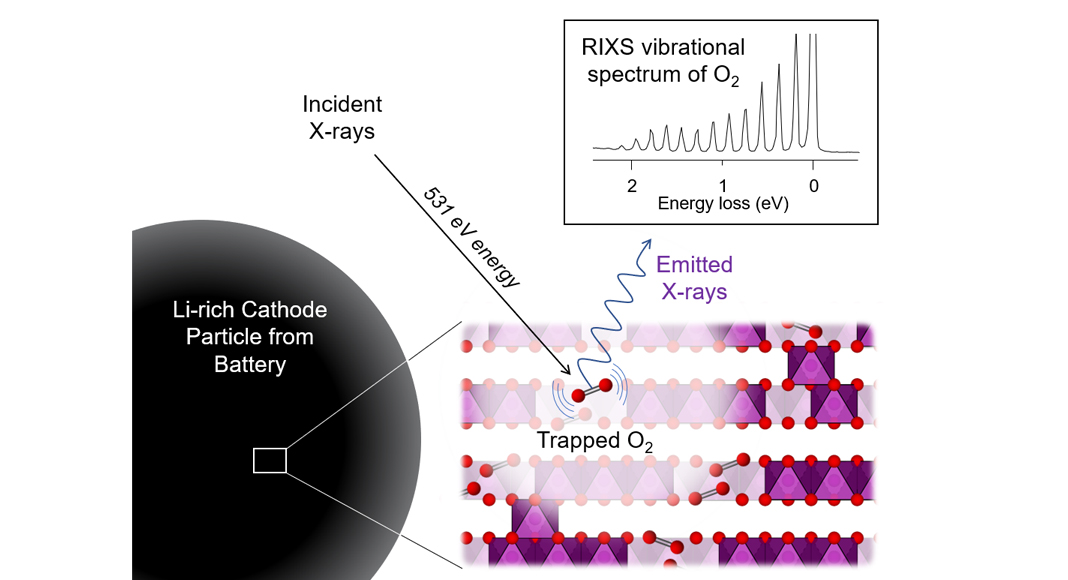
To characterise bulk redox processes, X-ray spectroscopy is the tool of choice requiring the use of high energy radiation which can penetrate through matter. To probe the changes to the electronic structure accompanying O oxidation and reduction directly, Resonant Inelastic X-ray Scattering (RIXS) has proven to be a particularly powerful spectroscopic tool. This permits direct measurement of the valence states on oxygen, where the electrons involved in the electrochemical reaction reside. Previous RIXS measurements at low resolution have shown a mysterious spectroscopic feature appearing at an excitation energy of 531 eV (O K-edge) in Li-rich materials which has become known as the ‘signature of O-redox’. This feature appears at the end of charge and disappears again on discharge, indicating it belies a reversible O-redox process, however, it has not yet been possible to conclusively assign its origin.
In our recent publication in Nature Energy, we studied the Li-rich cathode Li1.2Ni0.13Co0.13Mn0.54O2 with RIXS at a significantly higher energy resolution than before. This was made possible using the state-of-the-art capabilities of the I21 beamline at Diamond Light Source in the UK in collaboration with Principal Beamline Scientist Dr Ke-Jin Zhou and his team. The data revealed that the signature O-redox RIXS feature can be resolved into a series of sharp peaks arising from vibrational modes of O2 diatomic molecules. Since the RIXS measurements were performed under ultra-high vacuum conditions within the spectrometer, these O2 molecules must be trapped within the bulk of the cathode particles, unable to escape.
To confirm the formation and reduction of trapped molecular O2 by another experimental means, we employed solid-state 17O magic angle spinning nuclear magnetic resonance (17O MAS NMR) spectroscopy on cathode samples that had been isotopically enriched with 17O. The detection of a new, highly paramagnetic chemical environment for O during charge provided additional evidence to support to presence of molecular O2 in the cathode. Furthermore, using NMR, we were able to quantify the amount of O2 present and showed that it fully accounts for the extra capacity associated with O oxidation.
This striking result implicates molecular O2 as the primary form of oxidised O present in Li-rich cathodes. Our RIXS data further showed that this molecular O2 can be reduced back to oxide ions on discharge which reincorporate back into the structure and that this process occurs reversibly upon subsequent cycling. This new model to explain O-redox chemistry represents an important advance in understanding for this crucial class of compounds and is an unusual example of a heterogenous (solid-gas) redox reaction taking place reversibly in the bulk of a solid material.
The implications of O2 for battery performance
So, if most of the extra capacity is provided through the reversible formation and reduction of molecular O2, why is there such a large first cycle hysteresis? The answer to this question lies in the structural rearrangements that must take place to allow for molecular O2 to form in the first place. The Li-rich cathode material starts off with a highly ordered structure exhibiting a honeycomb-like pattern of Li and transition metal ions within each of the transition metal oxide slabs. When the lithium ions are removed during charge leaving behind vacant sites in the transition metal layer, oxide ions are oxidised at a high voltage. However, the honeycomb arrangement in the charged state is highly susceptible to disordering through transition metal migration which cannot be reversed. Our computational modelling reveals that this disordering is driven by the formation of molecular O2 which helps to stabilise the charged structure. It is this irreversible structural change that results in a substantially lower voltage discharge process in Li-rich cathodes.
While the overall energy density returned on first discharge is distinctly lower than the initial charge, it still represents an appreciable improvement on state-of-the-art cathode materials (around 30%), showing that O-redox involving molecular O2 formation may still be capable of delivering a much-needed boost in cathode energy density. Indeed, these materials are being hotly considered for next generation batteries to provide greater driving ranges for typical electric vehicles. However, these first-cycle changes also initiate a number of other problems which persist over cycling, such as residual voltage hysteresis and gradual voltage fade. More work remains to be done to uncover the origin of these features and mitigate against them, but the identification of molecular O2 as a key player in the redox chemistry of these compounds will certainly offer a firm platform for further understanding.
The discovery of molecular O2 in high energy cathodes and its role in voltage hysteresis also has important implications for the design of new battery cathode materials. To realise the full potential of O-redox, molecular O2 formation must be suppressed in order to achieve high voltages without first cycle hysteresis. We recently showed in a related article in Nature that first cycle hysteresis can be suppressed by making materials with a ribbon, rather than a honeycomb ordered, arrangement of lithium within the transition metal slabs. Ribbon ordering provides a more stable framework for O oxidation by suppressing transition metal migration and inhibiting the formation of molecular O2. This offers one possible new direction for the design of future O-redox cathode materials.
As the demand for higher energy density batteries from the automotive, aviation, and consumer electronics industries continues to intensify, we will increasingly need to turn to new cathode chemistries to achieve step changes in performance. The implementation of layered Li-rich cathodes in real-life commercial cells may still be hindered by practical challenges, but advances are continually being made particularly with new synthesis methodologies and surface modifications. The identification of O2 formation and its role in voltage hysteresis will inform and direct future material design efforts towards enabling O-redox technology in the next generation of Li-ion batteries.
The author of this article, Dr Robert House, a Postdoctoral Research Fellow at the University of Oxford’s Department of Materials, is the lead author on the recently-published paper in Nature Energy called ‘First-cycle voltage hysteresis in Li-rich 3d cathodes associated with molecular O2 trapped in the bulk’.
This paper reveals how a collaborative team of researchers have been able to fully identify the nature of oxidised oxygen in the important battery material – Li-rich NMC – using RIXS (Resonant Inelastic X-ray Scattering) at Diamond Light Source. This compound is being closely considered for implementation in next generation Li-ion batteries because it can deliver a higher energy density than the current state-of-the-art materials, which could translate to longer driving ranges for electric vehicles. They expect that their work will enable scientists to tackle issues like battery longevity and voltage fade with Li-rich materials.
The paper by a joint team from the University of Oxford, the Henry Royce and Faraday Institutions, and Diamond Light Source, the UK’s national synchrotron, examines the results of their investigations to better understand the important compound known in the battery industry as Li-rich NMC (or Li1.2Ni0.13Co0.13Mn0.54O2).
Please note, this article also appears in the fourth edition of our quarterly publication.