Innovation News Network outlines the variety of helium uses and explains how these uses contribute towards improving our quality of life.
Helium is a gas that has numerous applications in everyday life. From its use in medical treatments to providing buoyancy for aircraft and balloons, this element plays an important role in many aspects of our day-to-day lives. It is also the second most abundant element in the Universe after hydrogen and can be found on Earth primarily as a byproduct of natural gas extraction processes. This article will explore some common uses of helium and how they affect us all.
The first application of helium lies within the medical field. Helium’s unique properties make it ideal for Magnetic Resonance Imaging (MRI) scans and other imaging technologies used to diagnose patients with serious conditions such as cancer or heart disease. By using MRI technology, doctors are able to obtain detailed images of their patient’s internal organs without having to resort to invasive procedures which may cause discomfort or even harm during surgery. In addition, helium can be used to treat certain types of respiratory illnesses due to its ability to reduce airway obstruction caused by mucus build-up.
Finally, helium has been utilised extensively within aerospace engineering fields since its discovery in 1895. Its low density allows it to provide lift when contained inside lighter-than-air vehicles like hot air balloons or blimps; this makes them much easier to control than heavier crafts such as airplanes or helicopters. Helium is also commonly used aboard spacecraft where its low boiling point helps maintain optimal temperatures during long missions into deep space exploration activities.
Each use for helium contributes significantly towards improving our overall quality of life – from detecting deadly diseases early on through MRI scans, exploring distant galaxies via spacecrafts powered by helium fuel cells, or simply enjoying beautiful views from up high in a hot air balloon ride.
Properties of helium
Helium is the second-lightest element in the Universe and it has an atomic number of two. This makes helium a noble gas that is very abundant, especially on Earth. It can be found in the atmosphere at approximately five parts per million by volume, with most of its presence being in deep sea water or underground sources.
In terms of physical properties, helium’s boiling point is extremely low; -269°C (-452°F). Its density is also much less than air – 0.0001785 grams per cubic centimetre (g/cm3); making it lighter than other gases like hydrogen and oxygen. Additionally, this abundance means its specific heat capacity is quite high compared to other elements as well. So, when used in combination with other substances, it will help absorb more energy from them before reaching a temperature equilibrium.

The unique characteristics of helium make it valuable for many applications across science and everyday life, ranging from industrial processes to medical diagnostics and even recreational activities such as balloon flights or entertainment shows using special effects involving dry ice fogging machines.
Scientific applications of helium
Helium’s unique properties make it invaluable for several scientific applications. Its low boiling point and thermal conductivity at cryogenic temperatures make it an ideal coolant in the laboratory. As a noble gas, helium is also an inert gas that can be used to protect both humans and sensitive equipment from hazardous atmospheres in chemical reactions or welding processes. Researchers can use liquid helium as a cooling agent for specialised experiments such as nuclear magnetic resonance (NMR) spectroscopy and scanning tunnelling microscopy (STM).
These qualities enable scientists to study molecular structures, nanostructures, quantum effects, and other phenomena which require extremely cold conditions without risking damage to delicate instruments or harming personnel. Furthermore, helium’s non-toxic nature has made it useful for medical diagnostics such as MRI imaging technology. Without these capabilities provided by helium, much of our current understanding of the physical world would not exist today. It is clear that this element plays a vital role in many general scientific studies and more specific research projects alike.
Helium’s non-scientific applications
Helium is not only used in scientific and medical laboratories, it also has many practical applications that can be found in everyday life. It is a common component of cryogenics, the study of extremely low temperatures, as well as other industrial processes such as leak detection and fire suppression systems. Helium is particularly useful for these tasks because it does not react with other elements or molecules and its lightness makes it an ideal gas to detect leaks.
In addition to being a reliable gas for detecting leaks, helium is used as a coolant for certain types of magnets due to its ability to conduct heat away from sensitive components. This helps keep the temperature inside the magnet stable while still allowing electricity to flow through efficiently. Furthermore, helium allows welding cutting tools to work more precisely than they could without its presence by providing an inert medium that shields against oxidation during the process.
The most widely known use of helium in everyday life would be its role in inflating balloons; specifically those made out of latex or mylar which require much less effort when filled with this lighter-than-air gas. The buoyancy provided by helium gives the balloon lift making them perfect for parties, decorations, advertising, and even hot air balloon rides. In summary, non-scientific helium uses include:
- Leak detection;
- Fire suppression;
- Coolant magnets;
- Welding cutting; and
- Helium balloons.
These uses demonstrate how versatile and beneficial helium can be outside of research labs and hospitals.
Manufacturing uses of helium are varied and often essential
Helium is used in the production of many products, such as computer chips and semiconductor devices. It is also utilised for inert gas welding applications to join two pieces of metal together without burning or melting them. Additionally, helium is employed in various manufacturing processes, including superconducting magnets, which require extremely low temperatures; cryogenic freezing; and scanning transmission electron microscopy (STEM).
Helium’s unique properties make it an ideal choice for manufacturers looking to create high-quality products with precision and accuracy. Its ability to remain liquid at very low temperatures makes it useful for cooling down components that must be kept at a specific temperature in order to function properly. In addition, its lightweight and non-combustible nature allows it to be safely stored and transported within certain industrial settings.
Helium has become increasingly popular in recent years due to its use in creating gas-filled balloons and other novelty items. While these may seem like mere frivolities, they can actually provide important benefits when used in the right way. For instance, some retailers have begun using helium-filled balloons as decorations inside their stores, creating an inviting atmosphere that helps draw customers in. This indicates how versatile this element can be even outside scientific circles.
How is helium used in the medical sector?
Helium is increasingly used in medical applications. A study conducted by the National Institute of Standards and Technology (NIST) found that approximately 11% of all helium produced goes towards medical purposes, making it a top consumer for this element.
Medical uses include:
The ability to detect subtle changes allows helium to be employed in other areas such as cancer detection through MRI scans and even fertility treatments where the embryo’s development must be monitored closely over time. Similarly, scientists have developed ways to modify molecules’ behaviour using lower pressure environments filled with cold helium gas, thus allowing new proteins and enzyme structures to form while being studied under different conditions than those present on Earth’s surface.
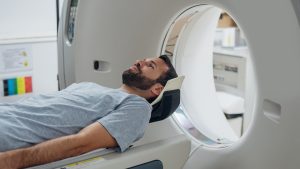
In addition, surgeons use liquid helium cooling systems during open-heart surgery procedures because they reduce blood damage caused by heat build-up around the heart muscle due to friction between instruments inside the patient’s chest cavity during operations. This helps ensure safety when performing delicate surgical tasks involving extensive manipulation of tissue within close proximity to vital organs. Lastly, its non-flammable nature also makes it popular in industrial settings where there could potentially be sparks from electric tools or welding equipment that may ignite combustible gases nearby which would otherwise cause explosions if present in large quantities.
Helium is an increasingly important element in industrial processes
Helium has a wide range of uses, with the most common being its use as a coolant production and storage medium. The low boiling point of helium makes it ideal for certain cooling operations that require temperatures lower than -268°C. Helium is also used in industrial welding, cutting torches, and leak detection systems due to its inert nature. As such, it can be found in many industries related to construction or manufacturing where these tools are necessary. Additionally, because helium does not react chemically with other substances, it is often employed in reaction vessels for laboratory experiments and research purposes.
The properties of helium make it an ideal tool for medical imaging procedures like MRI scans which rely on the element’s ability to absorb high-frequency sound waves. This allows doctors to get detailed images of organs inside the body without having to perform invasive surgeries or X-rays. Moreover, helium provides buoyancy in balloons and blimps when combined with oxygen and nitrogen gas mixtures; allowing aircraft designers the opportunity to create lighter airships than those made from traditional materials alone. In fact, this innovative technology has already been implemented by various militaries around the world that utilise mainly helium-filled surveillance blimps for monitoring activities over large areas of terrain.
Furthermore, NASA uses liquid helium extensively during launch operations since it acts as a powerful insulator against extreme cold temperatures at extremely high altitudes – all while keeping components stable under pressure changes induced during launches into outer space. Allowing scientists to better understand our Universe through deep space exploration missions enabled by liquid helium-insulated equipment is just one example of how this versatile element continues to shape new possibilities across multiple fields today.
Helium has a wide variety of uses in aerospace applications
Its low boiling point and light weight make it an ideal fuel for rocket propulsion systems. In addition, the inert nature of helium makes it safe to use as a propellant because it does not react with other elements like hydrogen or oxygen. This makes helium an excellent choice for fuelling rockets before launch.
The majority of space exploration missions require the presence of helium on board spacecraft due to its unique properties that allow high-pressure gas storage tanks to be filled without fear of explosion or fire hazards associated with more reactive fuels such as liquid hydrogen. Helium can also be used in combination with nitrogen to provide additional thrust during launch sequences and maintain pressure inside the combustion chamber of rocket engines while they are firing.
In addition, cryogenic liquids such as liquid nitrogen and liquid hydrogen must remain at extremely cold temperatures for efficient operation; this is where helium comes into play again. It can be used to cool down these liquids, so they retain their properties required for successful launches, providing a stable environment essential for operations carried out in outer space.
Helium uses in preservation
Moving on from aerospace applications, the uses of helium in everyday life are plentiful. From food preservation to gas preservation, there is no shortage of ways that this element can be used. To put it simply, helium has many different uses and serves a wide variety of purposes in our day-to-day lives.
The most well-known use for helium is probably its role in food preservation. By reducing oxygen levels within packages or containers, spoilage caused by bacteria growth can be greatly reduced or eliminated altogether. This process also helps maintain the flavour and texture of foods such as meats and fruits over time. Another common use for helium is artefact preservation – preserving items like ancient documents or artefacts from destruction due to aging processes. In addition, art galleries around the world often require controlled environment with low oxygen concentrations maintained by using helium gas to preserve artwork for long periods of time without any damage occurring.

Finally, one lesser-known application of helium comes in insect preservation – where specimens such as butterflies are collected and preserved in an inert atmosphere created through the presence of low levels of nitrogen and high levels of helium gas within sealed chambers. The benefits associated with this technique include minimal discoloration or fading over time and improved resistance against pests which may otherwise destroy delicate collections if left unchecked.
Helium is used in the production of radioactive isotopes
This process requires a nuclear reactor as well as specific radiation-shielding techniques that are utilised to contain and protect against ionising radiation associated with the production of radioisotopes. In order for successful production, it is important that all safety protocols are properly followed.
By utilising a controlled environment and correct precautions, radioactive isotope production can be safely conducted with minimal risk. The end product allows us to gain valuable insight into scientific research, identify medical problems more accurately, improve quality control processes, enhance safety standards, and ultimately make technological advancements possible.
Helium is used for detecting leaks
Helium gas is used for its unique physical properties, including being exceptionally light and non-reactive with other chemicals, which makes it ideal for detecting leaks in a wide range of industries. Leak detection using helium involves filling objects or systems with helium at a certain pressure and then looking for drops in this pressure due to any potential leakage points. If present, these leaking spots can be identified by utilising sensitive detectors like mass spectrometers and sniffers.
Helium’s low atomic weight and diffusion coefficient allow it to permeate most materials more quickly than heavier gases such as nitrogen and oxygen. In addition, because helium does not react chemically, unlike some other gases, it provides accurate readings without damaging the object being tested. Its inertness also prevents false positives from occurring, reducing the time required to identify possible leaks while increasing accuracy. Furthermore, since lighter molecules migrate faster through cracks than denser ones, leak detection using helium ensures rapid results even when testing complex structures or small components where conventional methods may struggle to detect faint traces of escaping gas molecules.
In today’s world, leak detection using helium plays an integral role in numerous engineering applications ranging from automotive engines to medical devices and consumer electronics among many others. It greatly contributes towards improving safety standards in industry by helping engineers locate defects quickly before they lead to major issues down the line. Indeed, thanks to its versatile nature coupled with reliable accuracy levels across various environments and scenarios, this technology is indispensable in modern-day industrial settings.
Coolant for superconducting magnets
Superconducting magnets are used in a variety of applications, from medical imaging and MRI scanners to particle accelerators. One important aspect of these devices is the need for cooling – superconductors must be cooled down to very low temperatures in order to function properly. A popular choice for this is helium, which has been found to provide an ideal coolant for magnetic superconductors. Helium’s low boiling point means that it can achieve extremely cold temperatures without freezing or condensing, making it suitable for use with sensitive equipment.

In addition, helium provides high thermal conductivity – meaning it is able to move heat away quickly and efficiently, allowing the temperature within the magnet to remain stable throughout its operation. This makes it an excellent choice when using powerful magnets as part of sophisticated machinery such as MRI machines and particle accelerators. The use of helium also allows for more efficient design; since there is no need for other forms of cooling systems like radiators or fans, designs can focus on optimising performance instead.
Helium’s unique properties make it invaluable in providing effective cooling solutions for magnetic superconductors and other delicate instruments. Its ability to maintain low temperatures while providing fast heat transfer makes it an indispensable asset in keeping magnets running at their peak efficiency. As technology advances and new uses are discovered for superconducting magnets, so too will our reliance on helium as a reliable source of cooling increase dramatically.
Welding and cutting applications
Helium has important uses for welding and cutting, due to its properties as a gas. It is used in arc welding processes by providing an inert atmosphere that shields the weld from oxidation or other atmospheric contamination. Helium can also be added to argon-based shielding gases when welding stainless steel, nickel alloys, and titanium, which improves weld quality while reducing porosity. In addition, helium provides greater penetration into thicker sections of material during the welding process.

Helium is also useful in oxyfuel cutting operations since it increases flame temperature. This characteristic allows metal fabricators to cut through materials more quickly and easily than with oxygen alone. When used at higher pressure levels, helium produces a wider cone of fire that increases cutting speed even further. Additionally, helium helps reduce carbon deposition on the surface of the part being cut so finished pieces have smoother edges after fabrication.
The use of helium for welding and cutting applications affords many advantages over traditional methods using air or oxygen atmospheres alone. It can help improve efficiency on production lines where precision parts require detailed attention during fabrication. The increased temperatures achieved by adding helium also allow manufacturers to work faster without compromising accuracy or safety considerations.
Helium has important uses in fire suppression systems
Using helium in fire suppression systems has become a common practice. The use of this inert gas to flood an area with the intention of suppressing flame and heat-producing fires is backed by scientific research and extensive experience from those who have used it successfully. Its non-toxic, odourless properties make it ideal for areas where human safety needs to be taken into account. Here are three ways that helium can help fight against fire:
- It acts as a barrier between fuel sources and oxygen, reducing the chance of combustion;
- It absorbs radiant heat energy, cooling down surfaces near the flames;
- It reduces smoke production due to its ability to expand quickly when heated.
When using chemical suppressants, such as foam or powder, they often leave behind residue which may require costly clean up procedures. However, with helium’s neutral composition, there is no need for additional clean-up efforts – making it more cost-effective than other methods of fire suppression systems. In addition, helium also works faster than traditional extinguishing techniques like water sprayers or carbon dioxide tanks because its molecules move much quicker through space. This means that once released into an affected area, the chances of containing a blaze are much higher compared to other options available on the market today.

Helium’s effectiveness in controlling fires makes it a popular choice for many professionals responsible for managing their premises’ safety measures. Not only does its inert nature guarantee minimal damage upon release but it also provides peace of mind knowing any potential risks have been mitigated – allowing businesses and public spaces alike to thrive without fear of destruction caused by flames or smoke inhalation.
Helium’s uses in everyday life are vital
The use of helium in everyday life is something that many people take for granted. Inexpensive and with no risk to human health or safety, it provides an important service within society. However, it can also have a significant environmental impact when not used responsibly. Helium is non-flammable and relatively abundant, but is not renewable – once gone, it cannot be replaced easily.
Being aware of the cost associated with using helium, as well as its potential risks and limitations, allows us to make informed decisions about how we use this precious resource. For example, limiting balloon releases into the atmosphere could help reduce the unnecessary depletion of this gas. We should always strive to find ways to conserve our resources while meeting our needs today without compromising future generations’ access to them.
It may seem like such a small thing – what harm can come from releasing just one helium-filled balloon? But if everyone takes part in conservation efforts such as these, then collectively we will be able to maintain the balance between consumption and preservation of helium for years to come. The irony here lies in the fact that although it may appear benign at first glance, helium plays an essential role in our lives which must be respected if we are to ensure its continued availability over time.










