The Karlsruhe Institute of Technology (KIT) outlines how, through cutting-edge calorimetric methods, they enhance the safety of post-lithium and sodium-ion batteries.
In the framework of the highly competitive Excellence Strategy competition of the federation and the federal states of Germany, the POLiS (Post Lithium Storage) Cluster of Excellence was started at KIT and Ulm University in January 2019. It has a budget of about €7 million per year and is scheduled for a duration of initially seven years. In this cluster, the Calorimeter Center at the Institute of Applied Materials – Applied Materials Physics (IAM-AWP) is responsible for the thermal characterisation and safety of post-lithium materials, electrodes and batteries. Post-lithium batteries use more abundant and environmentally friendly materials instead of Lithium, Nickel and Cobalt. These can be Sodium, Magnesium or Calcium. In the first three-and-a-half-year funding period, the focus was laid on the development of sodium-ion batteries (SIB), which are based on the same working principle as that of a Lithium-ion battery. Instead of lithium-ions, sodium-ions are transferred via an organic electrolyte through a separator between the two electrodes, in which they are intercalated and deintercalated, respectively.
Utilising advanced calorimetric methods
In an article in The Innovation Platform 6 last year,1 the first calorimetric results on coin cell level have been described. Using sophisticated calorimetric methods, progress in upscaling from coin cell to pouch cell level has been recently achieved. The cathode consists of the promising cathode material Na3V2(PO4)3 (NVP). The anode is made of commercial coconut-shell-derived hard carbon (HC) and the electrolyte of 1 M NaClO4 in EC:DMC:EMC (1:1:1) plus 2% FEC. NVP is a sodium super ionic conductor type material with high energy density, cycle stability, and rate capability, but a low electronic conductivity. Thus, in order to enhance the conductivity, porous NVP/C composites with carbon coating have been developed at the Institute for Applied Materials – Energy Storage Systems (IAM-ESS) to create an electronic conductive network.
The materials described above have been assembled into 5 cm ´ 5 cm pouch cells. Then the cells were formatted at C/10, reaching a capacity of 18 mAh and then cyclic aged at C/5 charge/discharge rate in a temperature chamber.
Unprecedented insights into sodium-ion batteries
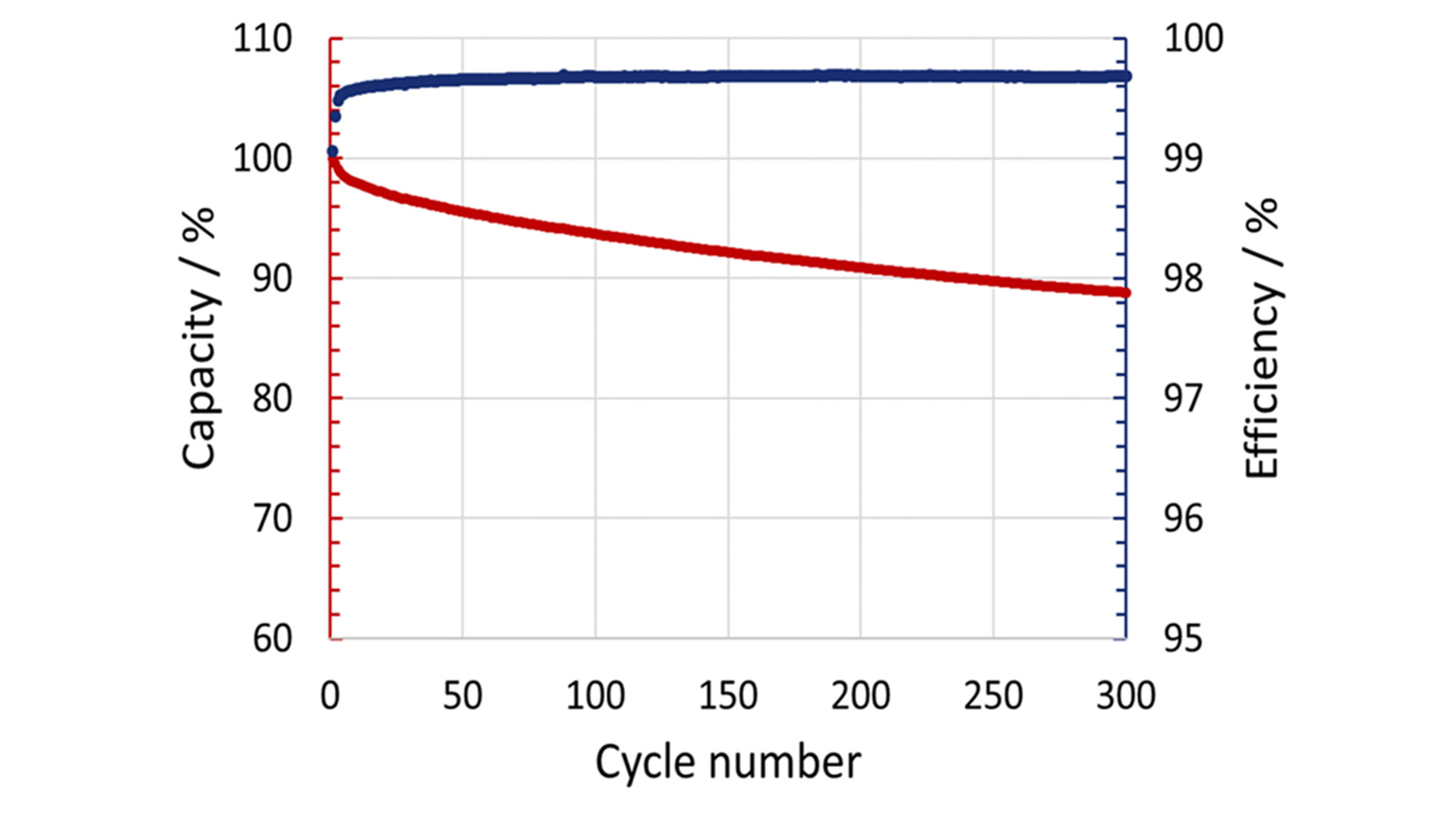
In the first five cycles, the capacity loss is relatively significant, whereas the coulombic efficiency increases to over 99% (Fig. 1). After this running-in period, they showed good cycling stability with a remaining capacity of 88 % after 300 cycles. The capacity reduction can be attributed to the loss of cyclable sodium. Besides the electrochemical characterisation, the characterisation of the thermal properties is needed to achieve an in-depth understanding of the underlying reaction mechanisms and heat conduction processes, which are, at the moment, not well-known for most post-lithium batteries. The generated heat during charging and discharging was determined using a highly sensitive MS80 3D Tian-Calvet calorimeter under isothermal conditions at 25°C. The heat flow is measured by the 3D Tian-Calvet Sensor arrangement, where both the sample and the reference vessel are surrounded by rings with hundreds of thermocouples.
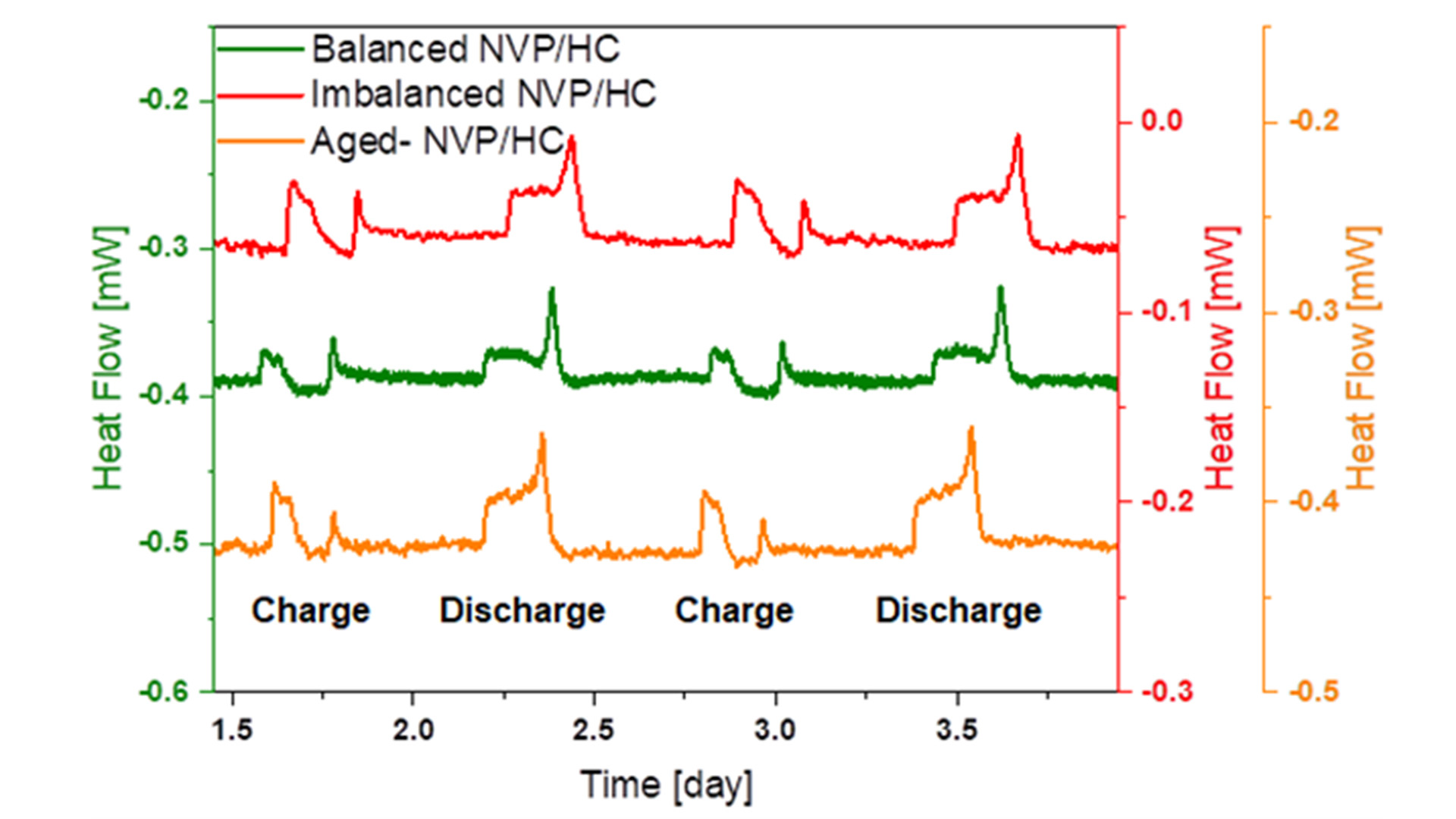
Because the pouch cells were too large to fit into the calorimeter chamber, fresh coin cells with 1 mAh capacity were assembled using the same materials. In addition, further coin cells have been made from extracted electrodes of the cyclic aged pouch cells with the same amount of fresh electrolyte as applied in the fresh coin cells. Fig. 2 shows the comparison of the operando heat generation curves for fresh and well-balanced (green curve), imbalanced (red curve) as well as cyclic-aged (orange curve) NVP/C vs HC full coin cells. During the calorimetric measurement, the cells were operated at a charge-discharge rate of C/5. In order to ensure that thermal equilibrium is reached, the cell was relaxed for ten hours between each charge and discharge cycle. It can be clearly seen that less heat is generated during charging than during the discharging process. In all cells, the heat generation of the cells becomes larger when they come close to a state-of-charge (SOC) of 100% and 0%. These pronounced peaks can be attributed to the higher internal resistance of the cell at these stages. In comparison to the imbalanced cell with an excess of anode material, the balanced cell shows a significantly reduced heat generation of approximately 50% for the charging and 20% for the discharging process, which is valuable feedback for the material developers and cell designers. The heat generation of the cycle-aged cell during discharging is even larger than that of the imbalanced cell. Additional investigations by electrochemical impedance spectroscopy and post-mortem SEM analysis revealed that the cyclic ageing process is predominantly driven by sodium plating on the HC electrode, which results in increased heat generation.
Thus it has been demonstrated that the measured heat profiles represent a fingerprint for the underlying electrochemical processes at the electrodes and are essential for SOH (state-of-health) and ageing prediction in sodium-ion batteries. This will help to pave the way for mature and safe sodium-ion batteries and other post-lithium batteries.
References
1. The Innovation Platform – Issue 6, pages 192-195