Scientists have developed a carbon nitride membrane that can help perform efficient lithium extraction from salt lakes.
The crystalline carbon nitride membrane, innovated by Qingdao Institute of Bioenergy and Bioprocess Technology (QIBEBT) researchers, could revolutionise the lithium extraction industry.
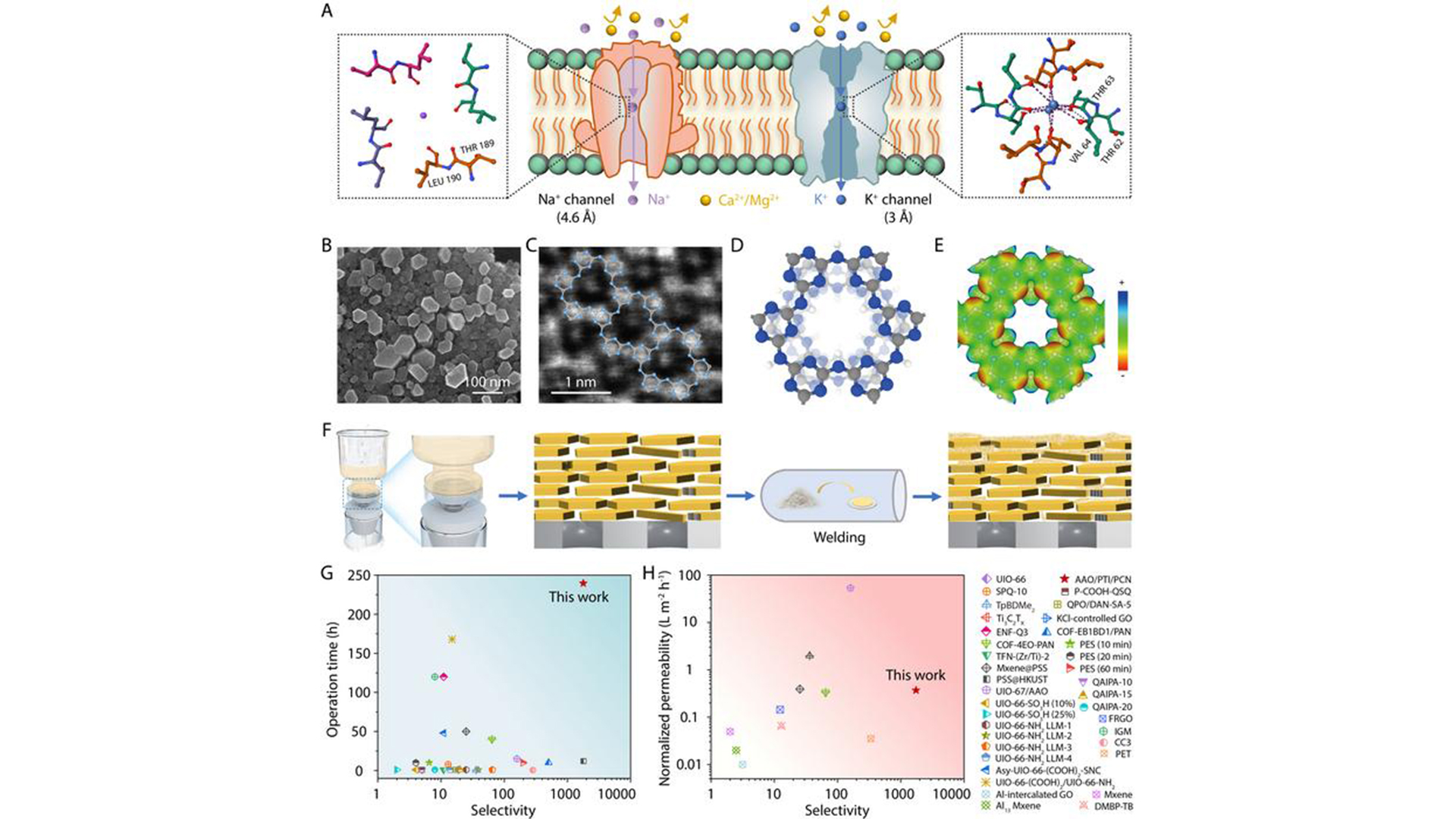
The technology mimics biological ion channels to expertly separate lithium ions from magnesium ions in salt-lake brine, demonstrating excellent efficiency and durability.
Advancing lithium extraction from salt lakes will be essential as the world strives to diversify its portfolio of lithium resources.
The demand for lithium will increase exponentially in the coming years as electric vehicles and renewable energy markets accelerate.
Professor LIU Jian, co-corresponding author of the study from QIBEBT, explained: “The advances achieved through this membrane technology offer new possibilities for efficient extraction of lithium, a crucial element in the transition to renewable energy and electric mobility.”
Carbon nitride membrane development
The membrane’s design draws inspiration from nature’s highly selective biological ion channels, which adeptly distinguish between various ions.
“We aimed to emulate these natural systems, developing a membrane that combines high selectivity with enhanced stability—essential features for practical applications,” explained ZHANG Yuanyuan, co-first author of the study from QIBEBT.
The membrane comprises a distinctive structure, which integrates both crystalline and amorphous forms of polymer carbon nitride.
This combination ensures uniform and narrow pores, effectively excluding larger hydrated magnesium ions while enabling smooth lithium-ion transport. This mechanism mirrors the seamless ion transport found in natural ion channels.
Credit: QIBEBT
Advancing salt lake lithium extraction
The bio-inspired crystalline carbon nitride membrane significantly outperforms traditional polymer membranes.
It achieves an exceptional selectivity ratio of 1,708, effectively extracting highly dilute lithium ions (0.002 M) from concentrated magnesium ions (1.0 M), a critical advancement for managing the high magnesium content in various lithium sources.
The team believes the new process could have uses beyond lithium extraction.
“The dual functionality of our membrane opens up new possibilities for its use beyond lithium extraction,” said professor GAO Jun, co-corresponding author of this study from QIBEBT.
“These properties could make a significant contribution to environmental protection efforts, in addition to improving the efficiency of resource recovery.”









