Professor Isabelle Opitz and Dr Michaela Kirschner explain the journey of developing novel treatment options for malignant pleural mesothelioma from preclinical studies to clinical trial.
Malignant pleural mesothelioma (MPM) is a rare but very aggressive cancer of the thin tissue layer (pleura) covering the lungs. The development of MPM is strongly linked to previous exposure to the fibrous mineral asbestos, which has been widely used until the 1980s, in particular in the building industry. Although patients are generally eligible to undergo multimodality treatment approaches consisting of combinations of surgery, chemotherapy, radiotherapy, and recently also immunotherapy, the prognosis for MPM remains rather dismal, with median overall survival reaching around 24 months.
Several factors are contributing to this poor prognosis, one of them being the difficulties associated with diagnosing MPM, which is difficult even for experienced pathologists, but even more so for general practitioners, who often only see a handful of cases during their career. Another reason for the poor prognosis is the intrinsic drug resistance of MPM, which is reflected in the fact that only approximately 40% of patients respond to the current standard chemotherapy of platinum + pemetrexed/gemcitabine. Furthermore, while surgical removal of the tumour is often part of the treatment concept, relatively early local recurrence within 12 months of tumour resection is a frequent issue that decreases the prognosis of patients and requires additional treatments to be added.1
Hence, for a disease like MPM, which is challenging in every step from diagnosis over initial treatment to subsequent treatment of recurrent disease, specialised centres are needed in which the patients are treated with individual concepts based on the decision of multidisciplinary tumour boards consisting of experts of various specialties (oncology, pathology, surgery, radiology, radiooncology, pneumology).
The Lung and Thoracic Oncology Centre Zurich (LTOZ)
At the University Hospital Zurich, the Lung and Thoracic Oncology Centre (LTOZ) is aiming to achieve exactly this: tailored treatment for each patient based on the latest developments and combined with translational research undertaken at the centre. The LTOZ is a certified centre for the treatment of lung cancer and other thoracic malignancies and combines daily clinical practice with translational research into various thoracic malignancies. At the LTOZ, more than 250 primary lung cancer cases are treated and the Department of Thoracic Surgery performs over 200 resections of lung cancer each year.
In addition, other thoracic malignancies are also being treated, including 30-40 cases of malignant pleural mesothelioma per year. With MPM having been a major research focus for many years, the Department of Thoracic Surgery has established a tissue database of all the patients being treated for MPM at the centre, and is currently extending this to NSCLC in collaboration with PD Dr A. Curioni-Fontecedro. Through this effort, we now have detailed clinical data of over 500 MPM patients and tissue samples of 200 patients, and Dr Felley-Bosco’s team at the Laboratory of Molecular Oncology in the Department of Thoracic Surgery has established 159 cell lines. Using these resources, we have assessed several clinical characteristics and biological factors for their potential prognostic role for MPM patients, and further studies to improve patient selection for treatment are on-going, as investigating and understanding the clinical, pathological, and molecular tumour characteristics is likely to allow the selection of optimal therapeutic approaches for individual patients.
In addition to these biomarker studies, we are also evaluating novel therapeutic options, both in the form of identification of novel molecular targets for MPM, and through the combination and refinement of currently-used treatment concepts. In recent years, this has led to the implementation of the INFLuenCe-Meso clinical trial (Intracavitary Cisplatin-Fibrin Localized Chemotherapy After P/D or EPP for Malignant Pleural Mesothelioma; NCT01644994).
INFLuenCe-Meso – from bedside to bench and back again
As mentioned above, early local recurrence is one of the major issues we face when treating MPM patients. This is at least in part a consequence of the anatomic situation (the pleura is covering the lungs and the chest wall), due to which it is not possible to resect the tumour with the usual safety margins of surrounding normal tissue. Therefore, it is likely that microscopic tumour remains behind, which most likely is the main reason for tumour recurrence not long after the initial tumour resection. To overcome this, and aiming to eradicate the remaining tumour cells, local therapies such as local irradiation can be performed. However, not all patients are fit enough to undergo this additional stress after major surgery. In addition, due to the underlying sensitive organs (lungs and heart), irradiation at high doses is difficult, and often associated with quality of life-reducing side effects.
We have therefore moved our translational research efforts from the bedside to the bench, aiming to improve local tumour control after tumour resection, using a localised therapeutic approach. The idea of applying intracavitary therapies, such as ‘washing’ with solutions containing chemotherapeutic drugs, had been implemented already in the 1980s, when for the first time patients with peritoneal cancers were treated with heated chemotherapy-containing solutions.2
The general aim of local intracavitary therapy is the application of the used drug at a high concentration on the area of interest, in combination with lower systemic drug concentrations and therefore lower systemic toxicity. For colorectal carcinomas and peritoneal mesothelioma (the less frequent form of asbestos-induced mesothelioma) intraperitoneal hyperthermic (heated) chemotherapy is a frequently used treatment modality.3;4 Unfortunately, when applying the same concept to pleural mesothelioma, despite promising results regarding time to progression, significant systemic side effects, in particular renal toxicity, has been reported.5; 6
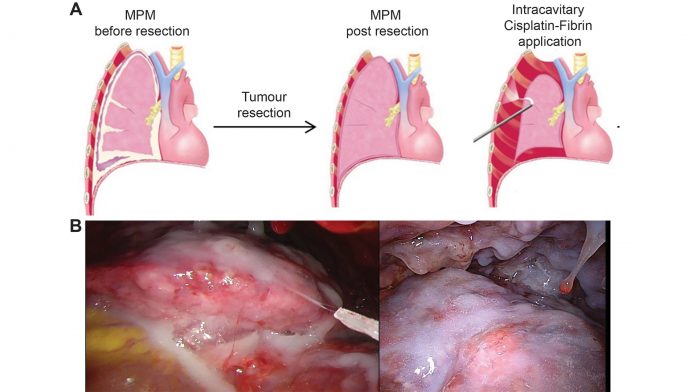
In order to overcome the issue of high systemic toxicity, possibly caused by a fast uptake of cisplatin from the heated wash solution, we have developed in our laboratory a novel concept by binding Cisplatin to fibrin (Vivostat®) used as a carrier. Fibrin, a natural glue in our bodies, can be produced from fresh frozen plasma, and using the Vivostat® System, this can be combined with your drug of choice and the resulting cisplatin-fibrin gel can be sprayed onto the desired area following tumour resection (see Figure 2).
When we tested this treatment concept in a specific small animal tumour recurrence model of MPM – developed only for this purpose – we observed reduced local tumour recurrence similar to the treatment with cisplatin-solution and, moreover, a better therapeutic index with much higher and prolonged concentration of cisplatin in the chest wall, while the systemic concentration was much lower.7;8 This was confirmed in a large animal model resulting in less side effects on kidney function.9
Following these very promising results, we moved back from the bench to the bedside, and undertook a Phase I clinical trial (INFLuenCe-Meso I), in which the safety and tolerability (effect on quality of life) of intracavitary cisplatin-fibrin after radical tumour resection was assessed (manuscript under review). The trial confirmed high local cisplatin concentrations in the chest wall even up to 70 days after the initial surgery, in combination with a high tolerability and systemic cisplatin concentrations below the known renal toxicity limit.
The safety of the highest dose applied in Phase I is now being further confirmed in the follow-up Phase II open INFLuenCe-Meso II trial, in which more than three quarters of the required patients have already been treated. In addition to its primary endpoints of safety and tolerability, the study will also allow us to gather information about the clinical efficacy of the treatment.
This trial is further complemented by a translational study investigating potential biomarkers that could predict treatment efficacy. Furthermore, in a closely-related small animal study we are again back at the bench, now investigating the feasibility, safety, and efficacy of combining intraoperatively administered intracavitary cisplatin-fibrin as radiosensitiser with adjuvant radiotherapy.
A subsequent Phase III trial to assess the efficacy of this novel intracavitary treatment concept is currently in the planning phase, in close collaboration between all involved partners of the LTOZ.
References
1 Opitz I. Management of malignant pleural mesothelioma-the european experience. J. Thorac. Dis. 6(Suppl. 2):S238–S252 (2014)
2 Spratt, J. S., Adcock, R. A., Muskovin, M., Sherrill, W. & McKeown, J. Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res 40,
256-260 (1980)
3 Sugarbaker, P. H. Surgical management of peritoneal carcinosis: diagnosis, prevention and treatment. Langenbecks Arch Chir 373, 189-196 (1988)
4 Sugarbaker, P. H. et al. Prospective randomized trial of intravenous v intraperitoneal 5-FU in patients with advanced primary colon or rectal cancer. Semin Oncol 12, 101-111 (1985)
5 Gomez, D. & Tsao, A. S. Local and systemic therapies for malignant pleural mesothelioma Curr Treat Options Oncol 15, 683-699
6 Sugarbaker, D. J. et al. Hyperthermic intraoperative pleural cisplatin chemotherapy extends interval to recurrence and survival among low-risk patients with malignant pleural mesothelioma undergoing surgical macroscopic complete resection. J Thorac Cardiovasc Surg 145, 955-963
7 Lardinois, D. et al. Intrapleural topical application of cisplatin with the surgical carrier Vivostat increases the local drug concentration in an immune-competent rat model with malignant pleuromesothelioma. J Thorac Cardiovasc Surg 131, 697-703
8 Opitz, I. et al. Local recurrence model of malignant pleural mesothelioma for investigation of intrapleural treatment. Eur J Cardiothorac Surg 31, 773-778 (2007)
9 Opitz, I. et al. Optimized intrapleural cisplatin chemotherapy with a fibrin carrier after extrapleural pneumonectomy: a preclinical study. J Thorac Cardiovasc Surg 141, 65-71)
Professor Isabelle Opitz MD, FEBTS
Senior Attending Surgeon and
Head of Research
Michaela Kirschner PhD
Research Co-ordinator
University Hospital Zurich
Department of Thoracic Surgery
+41 44 255 88 02
isabelle.schmitt-opitz@usz.ch
michaela.kirschner@usz.ch
www.thorax.usz.ch/
www.en.cancercenter.usz.ch/about-us/organ-centers/Pages/thoracic_oncology_center.aspx