By capturing the carbon from the furnace off-gases and converting them back into solid carbon feedstock, the Mecalo concept allows for renewable hydrogen to transform European production of critical raw materials like silicon and manganese.
Metals are vital to our modern way of life but, unfortunately, their production is also responsible for around 10% of global CO2 emissions. Even with increased focus on recycling of scrap and secondary resources, primary metal production is expected to grow in the coming decades. This increase is driven in no small part by the role of metals in the green transition, for use in wind turbines, solar panels, etc. As an example, an electric vehicle (EV) requires more than five times as many metals as its conventional counterpart.¹ If the green transition is to be successfully and sustainably implemented, it is necessary that metal production keeps up with the increasing demand, and that it does so while reducing and eventually removing fossil CO2 emissions. This is the motivation behind the Mecalo-Project, which was recently granted over €9m in funding from the European Commission to develop carbon-neutral processes for the production of metals, such as the critical raw materials manganese and silicon.
The journey to greener metal production
The metallurgical sector is often mentioned as one of the so-called ‘hard-to-abate’ sectors. The main reason for this, and why carbon-neutral metal production is so challenging, is that carbon contributes to the metal-producing processes as much more than an energy source and cannot simply be substituted by clean energy. This means that, even if society were to surmount the colossal task of completely converting the energy and electricity sectors to 100% renewable sources, that in itself would not be enough to decouple metal production from carbon. Metal production using today’s processes would still be heavy emitters.
To explain why that is, and to introduce the technological innovations of Mecalo, consider that metals are typically not found in their metallic form in nature, but that they are bound together with other elements in mineral ores. In many cases, including for manganese and silicon, they are found bound to oxygen. To liberate the metals, it is necessary to remove the oxygen, and this is where the carbon comes in.
In a simplified, generalised form, a metal-producing reaction can be written as a reaction between a metal oxide (MeO) and carbon, forming metal and CO2:
MeO + C → Me + CO2
Without carbon or another reducing agent, metal production is impossible. Typical efforts to decarbonise metal production thus revolve around one or more of the following:
- Replacing the fossil carbon reducing agent with sustainable carbon of biogenic origin.
- Capturing and storing the CO2.
- Replacing the carbon reducing agent with a non-carbon reducing agent such as hydrogen or electricity.
For the last point, the steel industry in particular has spent a lot of energy on developing hydrogen-based steelmaking. The advantage with hydrogen over carbon is that, in the metal producing reaction, the by-product of CO2 will be replaced by H2O:
MeO + H2 → Me + H2O
If the hydrogen used is produced without CO2 emissions, then the resulting metal production can be equally CO2-free. Unfortunately, while this approach is suitable for iron, several minerals will not react with hydrogen and give up their metals, as hydrogen is a less strong reducing agent than carbon.
The Mecalo concept solves this dilemma. Its main innovation lies in that it keeps using carbon as the reducing agent, but rather than releasing the CO2 into the atmosphere, it is recycled and reused within the process, in a principle known as carbon looping, outlined below.
How does the Mecalo concept work?
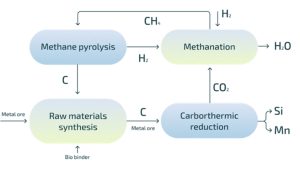
The Mecalo concept consists of four steps:
Methanation
The carbon dioxide in the furnace off-gas is reacted with hydrogen in a catalytic process.
CO2 + 3H2 catalyst CH4 + H2O
The hydrogen used is a combination of externally sourced hydrogen and hydrogen co-produced in the plasmalysis step.
Plasmalysis
Methane is unstable at high temperatures and will decompose into its constituents, hydrogen and carbon.
CH4 Energy Cs + 2H2
In Mecalo, this process is achieved with microwave plasma for maximum energy efficiency. The hydrogen that is co-produced with the solid carbon is returned to the methanation step in an internal hydrogen loop.
Agglommeration
Methanation and plasmalysis converts carbon from CO2 to solid carbon, but the result is a very fine powder akin to carbon black which is not directly suitable for metal production. A key point in the Mecalo value chain is therefore the agglomeration of this powder. The binder required to provide the agglomerates with sufficient strength will be of biogenic origin, maintaining the CO2-neutrality of the concept.
Metal production
The final step is to use our looped carbon material and demonstrate in pilot scale that it is suitable for metal production.
Hydrogen as a feedstock
Under the Mecalo concept, the core chemical reactions, those between metal ore and carbon, remain the same, creating metal as the product and CO2 as a by-product. But if we zoom out and view the entire process/flowsheet, we see that the material inputs are hydrogen and metal ore (and a small amount of bio-binder), and that the outputs are metal and water vapour. In other words, the Mecalo concept allows us to use hydrogen as a raw material in the production of metals where this has otherwise not proven to be possible. CO2 emissions from the core process are thus eliminated.
A big advantage of the concept described above is that, in principle, the core process stays unchanged. This means that there is no need for the complete overhaul or rebuild of existing smelter plants, greatly increasing the probability of rapid implementation and impact.
Further, as the concept is agnostic to the internal process, it can in principle be applied to any process that uses carbon as a raw material with CO2 as a by-product. For some processes, it may be easier to use the hydrogen directly. However, there are several metals – like silicon and manganese – where this is not an option. There will be a dedicated task within Mecalo looking at alternative applications.
Mecalo represents an innovative solution to a seemingly intractable problem: using hydrogen as a feedstock in metal production from ores that are thermodynamically unable to react with hydrogen. And this innovation does not rest on a recent advance only proven in the lab with years and years of development and scale-up before industrial implementation can be considered. Rather, it rests on the novel combination of four established technological principles. Thus, while research challenges certainly remain, there are no fundamental obstacles in basic research that need to be overcome. The individual steps of the four-step solution laid out above are all individually demonstrated at high maturity.
Methanation of carbon oxides is an important element in the production of synthetic natural gas from coal or wood, and is also used for CO/CO2-removal from process streams in ammonia production. There are no reasons why similar techniques cannot be utilised for the conversion of CO/CO2 in industrial off-gases into methane, but in real applications so far, however, similar efforts have typically focused on creating more high-value products, such as methanol, in carbon capture and utilisation (CCU) projects.
Similarly, the decomposition of methane into carbon and hydrogen has received a lot of attention, not least as a source of so-called turquoise hydrogen. A commercial plant is in operation in Nebraska, USA (Monolith).
Carbon black has been one of the alternative raw materials the metals industry has been investigating as a substitute for fossil carbon, as if it is sourced from bio-gas it could be considered CO2 neutral. In these trials, it has been demonstrated that methane can be used as a source of carbon materials fit for metal production.
The individual pieces of the puzzle are there, the work for Mecalo is to fit the pieces together and to optimise them in one overarching process. We are aiming to reduce scope 1 CO2 emissions from the production of silicon and manganese alloys by 95%. (The reason we cannot aim for 100% is that currently around 5% of CO2 emissions are due to the consumption of fossil-based carbon anodes, which is not within the scope of Mecalo. These last 5% could be eliminated by the use of anode materials of biological origin, or perhaps non-CO2-evolving anodes).

If successful, this could transform how metals are produced. It would allow Europe to be more self-sufficient on critical raw materials: 86% of EU demand for silicon and 50% of EU demand for manganese could be produced at near-CO2-neutrality in 2050, saving more than 33 million tonnes of CO2 emissions. This contributes to the EU’s net-zero goals on two axes – it greens the metal-producing sector while simultaneously providing the raw materials needed for the solar panels and windmills required in the Green Transition. In addition, the elimination of fossil coal in these processes could eliminate nine million tonnes of coal imports.

Mecalo brings together 12 partners from around Europe and is being co-ordinated by the Norwegian research institute SINTEF. The concept was first developed at Norwegian silicon producer Elkem, who in Mecalo is being joined by French manganese producer Eramet, who are also eyeing carbon looping as a strategy to decarbonise. The catalytic methanation process is being developed by JM in collaboration with SINTEF, while Sakowin Green energy are responsible for the plasmalysis of methane. ITPE in Poland and Glatt from Germany are the key partners working on agglomeration, while other partners are PDC (process modelling and optimisation) and BTG (bio-binder production) – both from the Netherlands, Ineris from France on safety, LIST from Luxembourg on LCA, and ETA from Italy on communication and outreach activities.
The project was launched in October 2024 and will run until March 2028.
This project has received funding from the European Union’s HORIZON Research and Innovation Actions under Grant Agreement No. 101177480. Views and opinions expressed are however those of the author(s) only and do not necessarily reflect those of the European Union or European Health and Digital Executive Agency (HADEA). Neither the European Union nor the granting authority can be held responsible for them.
References
Please note, this article will also appear in the 21st edition of our quarterly publication.