In recent years, drug discovery has been impaired by the lack of predictive models; microphysiological systems represent an excellent solution.
How many of the thousands of newly discovered molecules finally become drugs which effectively cure patients? Unfortunately, only a very low percentage (recently quantified around 12%). Why do so many candidate drugs fail before entering the market, although they show promising results at a research level? A recent paper indicates a lack of specific drug efficacy as the primary cause of trial failure in late phase development, failures that can be reduced by a more efficient screening of candidate drugs in early phases.
However, current 2D in vitro strategies, mostly based on cell lines cultured on 2D rigid substrates, represent the cheapest and easiest drug screening tool, but with major drawbacks as low-precision and un-natural environments. In vivo experimentation provides higher systemic physiological relevance, although associated to high running costs, increasing ethical issues and limited analytical depth. Moreover, animal models have been increasingly questioned about their power to faithfully reproduce human biological mechanisms.
Thus, alternative models better predicting the outcomes of new molecules in patients are desperately needed. Recently, innovative techniques such as microfluidics and tissue engineering have been emerging, aiming at reproducing the complex architecture and function of native human tissues. So-called ‘organs-on-a-chip’ or ‘microphysiological systems’ have recently been defined as “microfabricated cell culture devices designed to model the functional units of human organs in vitro”, thus representing an ideal platform for improving the predictability of drugs and biological therapies efficacy and safety in humans.
Our group believes that microphysiological systems hold the key to next generation health solutions. Following our 10+ years’ experience and specialisation in this area, we have emerged in the development of microphysiological systems in the musculoskeletal field, for platforms to study physio-pathological mechanisms and to perform reliable screening and testing of drugs for diagnostic and therapeutic aims.
In particular, these systems are used for currently uncurable bone diseases such as bone tumours and metastases, but also for pathological conditions of muscle tissue such as fibrosis, disabling pathologies (such as osteoarthritis), as well as ageing and metabolic diseases of the musculoskeletal system. This is achieved by reproducing human tissue districts focusing on high-fidelity biomimicking models through human multi-cellular and architecturally accurate models (i.e. whole joint model including all parts as bone, cartilage, synovium, vascular, immune in a single model).
Microfluidic Musculoskeletal models
The first example of a microfluidic, vascularised, human bone model for the study of bone metastatic invasion has been published on Biomaterials in 2014 and highlighted in The Economist journal. With this work, in collaboration with prof R Kamm from MIT, we were able to monitor the invasion of breast cancer cells in a bone-like matrix and the formation of micro-metastases in real time. In a subsequent work, we reproduced organotypic metastases from breast cancer, comparing engineered bone-like and muscle-like environments, and demonstrating the secretion of molecules in the muscle environment able to counteract tumour invasion.
To better mimic the metastatic process, blood and immune cells were also included in our recent models, demonstrating that the presence of blood cells (particularly platelet) increase metastatisation and that a drug used as antiaggregant clinical therapy can also decrease cancer invasion.
Microfluidic multi-tissue models have been also designed to investigate diseases affecting the joints, such as osteoarthritis.
A multichannel device, including the tissues of the native joint, as a cartilage compartment, separated from a compartment embedding synovial fibroblasts by a channel containing synovial fluid has been developed. The device mimicked the inflammatory processes at the basis of osteoarthritis and is being exploited to evaluate potential biological therapies, such as the injection of stem cells in the joint.
Microfluidics is a powerful technology however it comes with its own drawbacks too as the microenvironment into a microfluidic chip is not properly 3D, being able to host tissues with a thickness of just few cells (between 10 and 15), and thus also a scarce availability of biological material which makes it difficult to apply standard analytical techniques.
To overcome these limitations, in our lab we are exploiting both Micro- and Bio- fabrication techniques to generate miniaturised multicellular microphysiological systems, bigger and more user friendly than microfluidic ones which allow you to more accurately reproduce the 3D microarchitecture of native musculoskeletal tissues.
Musculoskeletal microscale models
In 2016 we generated a mm-scale construct, embedding osteoblasts, osteoclasts, vascular cells and calcium nanoparticles, to recreate the mineral part of the bone. This represented the first example of a bone-remodelling microscale model able to reproduce the balanced deposition and resorption of minerals by bone cells, recently further improved with the addition of macrophages. To test the potential of the device as a drug screening platform, we added breast cancer cells in the bone-like matrix and effects of different drugs have recently been tested. The model showed a significantly better reproduction of cancer cell resistance to drugs as compared to standard in vitro models.
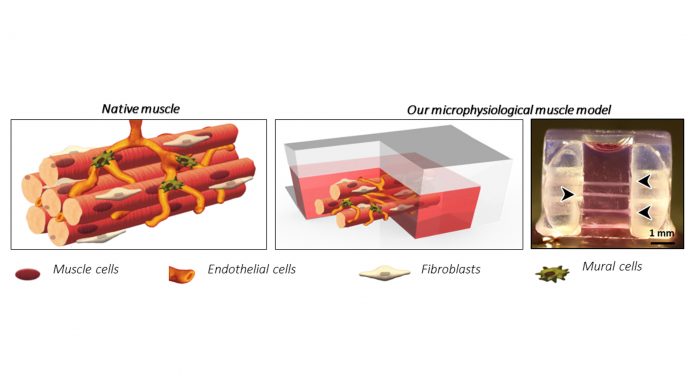
Beside bone, also a hierarchical microscale model of skeletal muscle has been described with multiple human muscle fibres engineered in a 3D gel. Here we showed for the first time the formation of the typical fibroblast layer surrounding each fibre intertwined with a microvascular network. Fibroblasts isolated from dystrophic patients and inserted in our model naturally exerted the traditional onset of fibrosis characteristics as compared to standard models requiring external induction for such behaviour.
To summarise, microphysiological systems represent the leading approach to achieve more reliable preclinical testing platforms for the quantification of drug efficacy, as compared to standard 2D models. However, further challenges lie ahead for their widespread use. In basic and translational research towards clinical application, a better understanding of pathophysiological mechanisms is mandatory.
Thus, faithful reproduction of complex native-like microenvironments should be achieved, including appropriate physical stimuli, whereby the exploitation of advanced microfabrication techniques can be of help towards this goal. Biofabrication of functional units of human tissues and organs is fundamental also for pharma companies, along with relevant automation and ease of use, to achieve more reliable readouts of novel drugs and highly predictive tests for biological therapies.
Depending on its final application, the complexity level of 3D in vitro models should be tailored to sufficiently improve relevance but without unnecessary additions and increasing costs. Anyhow, considering the multiple issues involved in the development of microphysiological systems, multidisciplinary expertise and knowhow in biological and bioengineering fields are mandatory, and fostering of translational researchers training will be needed to guarantee the emergence of such next generation systems.
Lastly, the huge amount of heterogenous data originating from such complex models need to be analysed with systems biology techniques, based on machine learning algorithms and similarly advanced techniques gathered from big data management. Fuelling research in these fields can help the research community in achieving better models of human organs, thus leading to drugs more effectively impacting patient care.
References
- J.N DiMasi, 2015. N Eng J Med
- C.J Karlsson. 2019. Pharmacol Exp Ther
- C. Williyard. 2018. Nature
- S.E Park. 2019. Science
- S. Bersini, and M. Moretti. 2014. Biomaterials
- https://www.economist.com/science-and-technology/2014/02/15/secondary-goals
- J. Jeon, and M. Moretti. 2015. PNAS
- C. Mondadori, and M. Moretti. Osteoarthritis and Cartilage
- M. Bongi and M. Moretti. 2016. Nanomedicine (Lond)
- S. Bersini and M. Moretti. 2018. Cell Rep
Matteo Moretti
Head
Regenerative Medicine
Technologies Lab
Unità di Ortopedia e Traumatologia, Ente Ospedaliero Cantonale
Lugano (CH)
+41(0)918117076
Matteo.moretti@eoc.ch
https://www.eoc.ch/