Dr Christophe Drouet and Dr David Grossin explain the relevance of calcium phosphate-based materials for bone repair and nanomedicine.
For decades, nature has inspired civilisations throughout the world. Researchers have often attempted to mimic natural processes and materials to fulfil human needs, based on concepts that have taken millions of years to establish. In this view, calcium phosphate-based materials (such as nacre, bone, teeth, coral, and antlers) have attracted a lot of attention as a result of surprisingly-high bioactivity, mechanical properties and/or complex three-dimensional (3D) organisations. Also, interestingly, this is despite some very soft conditions of formation in terms of temperature, pressure and pH levels.
Why biomimetic calcium phosphate?
One particularly appealing compound found in biominerals is nanocrystalline apatite (NCA), alongside its potential precursors such as amorphous calcium phosphates ACPs and octacalcium phosphate OCP. NCA is a calcium phosphate constituting the mineral part of bones and teeth.
It is now possible to prepare biomimetic synthetic analogues by following bio-inspired methodologies, therefore opening a wide medical exploration field. Besides the intrinsic biocompatibility of NCAs (they can mimic bone apatite, making them particularly suitable for the setup of implantable biomaterials and medical devices), other properties are also appealing for their use in medicine.
In particular, the surface of apatite nanocrystals contains very mobile ions which can be easily involved in ion exchange processes. This is naturally used by all vertebrates to maintain homeostasis in body fluids and can be exploited to produce biomaterials with tailored properties. For instance, an enrichment in copper or silver ions confers antimicrobial properties, while magnesium and strontium ions can modulate the activity of bone cells. A wealth of biologically-active cations and anions can therefore be associated to the nanocrystals to convey new functionalities.
In parallel to this, the high surface reactivity of the nanocrystals allows for the surface functionalisation/adsorption of a large variety of molecular organic species that are relevant to biomedicine. Again, these surface properties are often related to the presence of mobile surface ions that can take part in such adsorption processes. Examples of active (bio)molecules or drugs that may be associated to NCAs are antibiotics, anticancer drugs, antimicrobial enzymes, haemostatic drugs and cell-addressing agents. These surface capabilities are linked to the very specific surface features of apatite nanocrystals which do not exist in other ubiquitous mineral systems. Indeed, the nanocrystals are covered by a so-called ‘hydrated amorphous ionic layer’ which is not present in well-crystallised stoichiometric hydroxyapatite (Ca10(PO4)6(OH)2), and whose specific features have already previously been identified and discussed.1
From apatite nanocrystals to 3D biomaterials
Biomimetic NCA nanocrystals are not only lab curiosities, they can be consolidated into bulk (possibly porous) implantable biomaterials by way of non-conventional approaches such as cold sintering by Spark Plasma Sintering (SPS).2 The intrinsic presence of water and mobile ions on the nanocrystals surface enable diffusion processes at much lower temperatures than what is usually required for ceramics, which allows retaining the appealing features of NCAs even after consolidation.
The possibility to further modulate the mechanical properties of obtained scaffolds was also demonstrated via the association with (bio)polymers. It is also possible to prepare injectable bone cement formulations that harden in situ, in the implantation site, and which lead to bone-like apatite after final cement setting.3 Another approach is developed by coating metal or ceramic prosthesis/implants with NCAs which is feasible by using soft processing routes to maintain the hydrated and nanosized characters of the initial NCA crystals. For example, this is achievable by approaches such as electroprecipitation from calcium and phosphate sources in controlled conditions, by dip-coating in apatite gels, or by cold-spray of NCAs powder.4
The high versatility of biomimetic apatites in terms of possible associations with (bioactive) ions and molecular species is particularly suitable for medical uses. One domain of application is bone repair (a final goal being tissue engineering) as NCA represents approximately 70% of bone mass. This is all the more relevant that NCAs resorption in vivo can be modulated by their conditions of preparation (which dictate the thermodynamic stability of the apatite phase and thus its solubility); and also considering the possibility to tailor their physical-chemical and biological properties depending on their chemical composition.
Addressing health issues like bone infection
Among the variety of universal health issues that need to be faced nowadays is the fight against infections. This is especially problematic in bone repair cases as the high porosity of bone tissue makes the eradication of an established microorganism-bound infection rather delicate. Instead, the prevention of infections and the destruction of pathogens right after implantation (possibly with a prolonged effect) is a more sensible scenario.
To this aim, the combination of NCA-based biomaterials with antimicrobial ions and/or molecular agents is a relevant approach which is made possible by the immense potentialities of biomimetic apatites in terms of surface reactivity and derived tailorable bioactivity. This, associated to the design of original porous 3D scaffolds, is one of the solutions envisaged by the AIMed European project.5 One relevant point is to use bioactive ions insofar when possible, allowing a dose reduction of drugs such as antibiotics which are known to lead to resistance mechanisms in bacteria. In addition to tailoring bone regeneration around the implant, developing alternative antimicrobial strategies to antibiotics is one of the investigation points of this H2020 EU project.
Other challenges in this field include controlling the local healing of bone tissue and orienting cellular activity. This should allow progressing toward more personalised medicine and the development of smart release systems, which represent two major objectives for tomorrow’s medicine.
Apatite nanoparticles: a multifunctional platform for nanomedicine
Besides bone repair, the intrinsic biocompatibility of bioinspired apatites and their tailorable functionalities can be developed in view of other original, sometimes pioneering, applications (such as medical imaging or for tissue/cell therapy).6
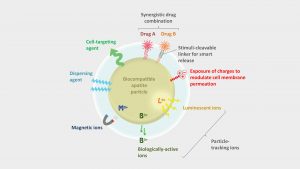
The nanometre dimensions of the particles associated to their versatile composition and adsorption capabilities, allow for the development of applications in nanomedicine. Examples were initiated in the fields of oncology (e.g. to target and kill remaining cancer cells after tumour surgical extraction), and more recently, in haematology (e.g. for the cryoprotection of red blood cells) or in dermatology (e.g. we developed in our group a strategy for the treatment of acne and now address other skin disorders) and other potential uses may still be developed. In this view, bioinspired apatite nanoparticles (NPs) can be seen as a genuine multifunctional platform for (nano)medical applications (see Fig. 1).6
Research trends
In our research group (‘Phosphates, Pharmacotechnics, Biomaterials (PPB)’) at the CIRIMAT institute in Toulouse, we seek to understand the underlying mechanisms in the synthesis and functionalisation of calcium phosphate-based materials apatites and their possible calcium phosphates precursors. We are also designing original processing approaches by soft chemistry to obtain scaffolds while retaining their appealing hydrated and nanosized features.
This is associated to a thorough characterisation of the obtained compounds via multiple complementary techniques that are necessary in order to distinguish different calcium phosphates and to identify bulk and surface features. We also examine the applicative potentialities of such mineral or related mineral/organic systems for biomedicine from a very large viewpoint, ranging from bone repair to nanomedicine to modulate the activity and/or treat cells or tissues. This includes numerous studies of functionalisation/adsorption with active ions and (bio)molecules/drugs.
Several systems nowadays, may show limitations on several aspects relating to an uncertain biocompatibility (including that of generated metabolites), a poor resorption rate in vivo, a less versatile chemical composition, and/or the less-controllable adsorption of active molecular agents. Plus, in bone sites, the implantation of other materials generally leads to the formation of a carbonated apatite layer upon precipitation from body fluids (that are naturally supersaturated relatively to hydroxyapatite): therefore, directly formulating apatite implantable constructs appears as a more direct approach, and their composition and physical-chemical features can then be finely designed from the start.
Bio-inspired apatites and related calcium phosphate-based materials therefore appear to be particularly relevant for a large panel of biomedical applications; and should lead to the development of other smart medical devices in the future.
The AIMed project has received funding from the European Union’s Horizon 2020 research and innovation programme under the Marie Skłodowska-Curie grant agreement No 861138.
References
- Drouet, C. et al. 2018. Nanocrystalline apatites: The fundamental role of water. Am. Miner. 103;550-564
- Grossin, D. et al. 2010. Biomimetic apatite sintered at very low temperature by spark plasma sintering: Physico-chemistry and microstructure aspects. Acta Biomat. 6;577-585
- Jacquart, S. et al. 2013. Composition and properties of silver-containing calcium carbonate–calcium phosphate bone cement. J. Mater. Sci.: Mater. Med. 24;2665-2675
- Kergourlay, E. et al. 2016. First cold spraying of carbonated biomimetic nanocrystalline apatite on Ti6Al4V: physical-chemical, microstructural, and preliminary mechanical characterizations. Adv. Eng. Mater. 18;496-500
- http://www.aimed-itn.eu
- Drouet, C. et al. 2020. Colloidal apatite particles: a multifunctional platform in (nano)medicine. Juniper Online J. Mater. Sci. 6(1); art.555676;1-8
Christophe Drouet, PhD
Head of PPB group
christophe.drouet@cirimat.fr
David Grossin, PhD
Vice Head of PPB group
david.grossin@ensiacet.fr
CIRIMAT Institute
University of Toulouse / UMR CNRS-INP-UT3
+33 05 34 32 34 11
+33 05 34 32 34 20
www.cirimat.fr
Please note, this article will also appear in the second edition of our new quarterly publication.