Neural Engineering is a new discipline at the interface between engineering and neuroscience; Dr Durand’s research combines computational neuroscience, engineering and electrophysiology to solve problems in the central and peripheral nervous systems.
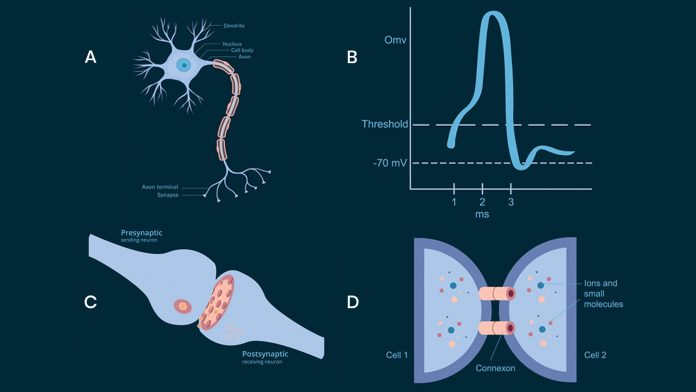
Neurons in the brain communicate by sending action potentials, low amplitude voltage signals that propagate along axons to a target cell (see Fig.1 A,B). At the junction between the two cells, called a synapse (see Fig.1 C), the incoming action potential releases a neurotransmitter to activate the downstream neurons. The process is called chemical synaptic transmission and is by far the most common type of communication between neurons and underlies all of the brain functions.1 However, neurons can also communicate through gap junctions between two cells (see Fig.1 D). A direct electrical connection is formed between the two neurons and therefore this process is called an electric synapse.2 The gap junction allows ions and electrical impulses to directly pass through a regulated gate between cells connecting the intracellular compartments of the two neurons.
Although fast, the role of gap junctions in neural function has not been clearly identified. Yet another method of communication is thought to take place through the process of diffusion. Molecules released by one or several cells can diffuse through the extracellular space and activate other neurons. Potassium ions and gases such as nitric oxide are well known examples of diffusion mediated communication.3,4 However, this process is significantly slower that synaptic transmission by several orders of magnitude. Finally, neurons generate electric fields associated with currents passing through membrane channels. These electric fields can be recorded (i.e EEG) but are very small and not thought to be large enough to activate other neurons. Yet neurons have been observed to influence each other through weak electric fields, a process known as electric field coupling or ephaptic coupling.5,6
Can neural activity travel without synapses?
Waves of neural electrical activity have been recorded in the brain and have been associated with brain function or state. The current understanding is that spontaneous brain rhythms originate from individual neuronal currents and their interactions with other neurons through chemical or electrical synaptic transmission. Non‐synaptic influences via exogenous or even endogenous electric fields (e.g. ephaptic mechanisms) have been suggested to play a role in modulating ongoing neural activity but are thought to be too small to activate other neurons.7 However, it has recently been shown that wave activity can propagate non-synaptically in the hippocampus.8-10
In particular sleep waves, as well as epileptiform waves, can propagate in the hippocampus by a mechanism consistent with electric field coupling.11,12 These self-propagating, non-synaptic waves can propagate with steady amplitude and direction in the hippocampus. They can propagate through a physical cut in neural tissue and therefore these hippocampal waves are self-regenerating via endogenous electric field coupling. Experimental results show that electric field coupling is both a necessary and sufficient mechanism that underlies the brain waves propagation in vitro.11,12 These results also show that endogenous electric fields, previously thought to be too small to activate neurons, can lead to the generation of self-propagating waves.
How can neural waves propagate by themselves?
Action potentials propagating at constant amplitude along axons are self-propagating as the incoming wavefront activates the sodium channels located in front of the wave producing another action potential and therefore generating an advancing wavefront. Waves in in the brain are thought to arise from the underlying neuronal activity and do not always follow axonal pathway (white matter tracts). They are not thought to be self-propagating since they are a reflection of and dependent on the neural dendritic process which produces them.
However, our experiments show that neural waves can propagate along the longitudinal axis of the hippocampus while maintaining their amplitude in the absence of synaptic transmission or gap junctions at a speed of about 0.1m/s (this process is illustrated in Fig. 3). This activity in a horizontal hippocampal slice preparation occurs through dendritic NMDA receptor‐dependent Ca2+ spiking. Consistent with purely electrical field coupling, this activity and its active propagation across the slice are resistant to pharmacological blockers of fast ionotropic chemical neurotransmission, as well as pharmacological blockade of electrical transmission via gap junctions.
Ephatic coupling vs. electric field coupling
Ephaptic coupling is a form of communication between neurons. From the Greek word ‘ephapsis’ (meaning to touch), refers to a neural element from one cell located very close to another cell thereby forming an ‘ephapse‘. The ionic current flowing in or out of a neuron enters another nearby cell and generates depolarisation, or hyperpolarisation, of its membrane. This mechanism of interaction between two cells is distinct from and independent of either electric or chemical synaptic transmission. It has been observed to play a limited but well documented role in the interaction between cells in vertebrate brains.13 Moreover, because this mechanism of interaction involves currents and electrical fields only, this effect is also called electric field coupling. Activation of many neurons can generate endogeneous electric fields capable of synchronising other neurons in mammalian brain.7
While ephaptic coupling is limited to highly localised connections between neural elements, electric field coupling can have a larger spatial extent. While non‐synaptic effects by electric field coupling or ephaptic mechanisms have been suggested to play a role in modulating ongoing activity, these effects are thought to be somewhat limited, at least during physiologically relevant activity.7
Can neural activity propagate through a complete cut of the tissue?
Neural events or waves have been observed to propagate across neural tissue but are not expected to cross a cut or gap in the tissue since axons would be damaged rendering the synaptic transmission non-functional. However, experiments carried out in vitro, whereby a thin slice of the hippocampus is maintained alive within the specialised chamber showed that neural events recorded by voltage sensing electrodes can propagate without synaptic transmission or gap junction through a complete cut of the tissue.12
An experimental cut to test this hypothesis would eliminate axonal pathways, remove gap junctions and synaptic transmission simultaneously. In vitro experiments carried out in the hippocampus confirmed that indeed neural events are observed crossing a gap in in the neural tissue as long as the gap is not greater than 400um (see Fig. 4).11,12
The speed of the propagation with and without the gap remains the same around 0.1m/s. This surprising result is consistent with the fact that electric fields propagate within a conducting medium near the speed of light and there is therefore a negligible delay added by the gap itself. The results of these experiments clearly show that weak endogenous field, previously thought to be too small to activate other neurons, appear to be solely responsible for the self-propagation of neural events and therefore must generate electrical fields large enough to activate other neurons.
How can electrical activity propagate through areas with no neurons?
Neural electric fields are generated by current sources and sinks produced by membrane ionic channels. They are described by a simple version of Maxwell equations called the quasi–static formation since the largest frequency of recorded voltages is below 10KHz.14 These fields can propagate with known attenuation within volume conductors such as the conducting solution bathing the neural population.14 Therefore, it follows that the electric fields generated by neurons should also propagate through a complete cut of the tissue provided the gap is short enough to minimise attenuation. Neurons however are needed to sense the electric field and generate currents and field to activate other cells nearly and resume propagation.
If electrical fields produced by neurons can activate the neighbour cells, then applying electric fields with similar amplitude should also generate waves at similar speeds and amplitudes. Applied electric stimulation to the tissue with currents producing electric field amplitudes similar to amplitudes observed during propagation did indeed generate similar waves recorded by two electrodes were positioned in the cell layer of the hippocampal slice.12 The amplitude of electric fields generated was not significantly different to 4-AP spontaneous activity (4.99 ± 1.14 mV/mm). The waves initiated by the applied electric field propagated with a propagating speed of 0.09 ± 0.03 m/sec similar to the speed of events occurring spontaneously.
Cancelling the electric fields can abort the propagation
It is proposed here that the electric field alone is responsible for propagating the neural activity. Therefore, cancelling the electric field generated by neurons should stop an incoming wave if the mechanism of propagation requires an electrical field. This experiment can be implemented by applying locally an electrical field of equal amplitude and opposite polarity to that of an incoming wave. Neuroscientists use a method called voltage clamp to force the membrane at a fixed potential and measure the resulting current by placing an electrode intracellularly.
We developed a similar technique to control the extracellular electric field with a clamping circuit capable of measuring the field of an incoming wave extracellularly. A current is then applied also extracellularly with a different set of electrodes to set and maintain the electrical field to zero. Experiments carried out with this electric field clamp technique showed that the neural events could propagate up to the clamp site but were completely stopped at that same location.12
Removal of the clamp allows the events to propagate again indicating that there was no damage to the tissue. This effect could be controlled by changing the gain of the feedback gain of the clamping system indicating a direct effect on the endogenous field generated by the neurons. In addition, the clamp did not affect the amplitude of the events distally suggesting the clamp effect is local and does not affect the source of the incoming wave.
How can neurons communicate by electric fields?
The mechanism of action of electric field coupling can be best understood by simulating the effect on a computer. Computer models of neural networks that retain the essential elements to a particular phenomenon can be built to test the mechanism of propagation. A simple network of 200 (x-dir) by 18 (z-dir) neurons was built with neurons communicating only by electric fields.9,11,12 Each cell has a soma and dendritic compartments (y-dir) but no axons or synapses. Each compartment is sensitive to the external electric voltage and electric field and the electric field at each compartment of every cell is computed by using the simplified Maxwell equation for isotropic and homogenous media.15
Activating a group of cells could generate local activation and induce a neural event similar to those observed experimentally. However, more interestingly, these events were observed to propagate along the array (x-dir) at the speed nearly identical to the speeds observed experimentally. These results indicate that electric field coupling whereby the ionic channels located within the neural dendrites of neurons can generate electrical fields that activate other cells and generate a self-propagating waveform at constant speed and amplitude. The effect of the electrical field cancellation and cut could also be reproduced in silico. The computer simulations predict that decreasing or increasing the extracellular volume should speed up or slow the speed of the propagation. This prediction was tested experimentally changing the osmolarity of the solution around the neurons. Decreasing osmolarity causes neuronal swelling, thereby decreasing the extracellular and increasing speed. Similarly, increased osmolarity decreased the sped of propagation.9
Relevance of electric field coupling
Waves propagating at speeds similar to those studied here have been observed in human and animal’s brains. For example, theta waves in cats propagate along the hippocampus at about 0.1m/s.16 Before and during epileptic seizures, neural spikes have been observed to propagate also at around 0.1m/s.17 The speed similarity obtained from very different recording conditions suggest a common mechanism such by electric field coupling. Indeed, our recent experiments have implicated these endogenous electrical fields in the recruitment of neurons during seizure-like activity in vitro following a block of neural transmission.11,18
In addition, both interictal spikes and seizure-like events were observed to propagate through a complete cut of the tissue. Hippocampal transections are currently used by neurosurgeons to isolate parts of the hippocampus and prevent seizure spread.19 However, the results above indicate that a seizure can go through a cut explaining why these procedures can fail in some patients.20 In addition, these results indicate that the electric field coupling could play a significant role in seizure generation and propagation thereby leading to novel therapeutic treatment option.
Electric field coupling could also play an important role in neural recruitment during normal brain function. In particular, brain waves recorded by EEG in subjects during deep sleep (slow wave sleep) can be reproduced in experimentally in vitro. These waves share similar shape and speed of propagation with the events described above.21 These deep slow sleep waves were shown to propagate non-synaptically, could not be blocked by either presynaptic junction blockers or gap junction blockers and could propagate in vitro through a complete cut.11 Although is not clear what their role is in the brain, they are now believed to participate in some the function related to memory consolidation known to take place during sleep suggesting a potential functional role of electric field mediated waves in memory.
Neural communication with synaptic transmission and gap junction has been well established and the molecular processing involved is known. It is also well known that neural activity produces currents, electric and magnetic fields that can be recorded with appropriate detectors. The neural induced magnetic fields are extremely small (in the 10-12fT range) and cannot generate neural activity. However, electric fields generated by neural activity (1-5V/m) have been reported to be able to modulate neural activity but not to generate excitation. The reason for this lack of ability to excite neurons is that amplitude is thought to be below the threshold for activation.
However, it now seems clear that endogenous electric field are capable of activating neurons through electrical field coupling. This form of communication between neurons can sustain neural waves that are self- propagating in the hippocampus without synaptic transmission and also in the cortex (unpublished data). The role of the waves is as yet unclear but some brain waves such as theta waves known to propagate at similar speeds are known to be involved in encoding information in the hippocampus. Therefore, a wave propagating by a non-synaptic mechanism could function as a timing signal for neural plasticity without interfering with synaptic weights.
References
- 2000. Principles of neural science – KANDEL. MCGRAQ-HILL COMPANIES 91
- V.L Bennett, and R.S Zurkin. 2004. lectrical Coupling and Neuronal Synchronization in the Mammalian Brain. Neuron 41, 495–511
- Nicholson. 2001. Diffusion and related transport mechanisms in brain tissue. Reports Prog. Phys. 64, 815–884
- Li, and C. Iadecola. 1994. Nitric oxide and adenosine mediate vasodilation during functional activation in cerebellar cortex. Neuropharmacology 33, 1453–1461
- G.R Jefferys., J. Deans, and M. Bikson et al. 2003. Effects of weak electric fields on the activity of neurons and neuronal networks. Radiat. Prot. Dosimetry 106, 321–323
- T Francis., B.J Gluckman, and S.J Schiff et al. 2003. Sensitivity of Neurons to Weak Electric Fields. 23, 7255–7261
- Anastassiou., R. Perin, and H. Markram et al. 2011. Ephaptic coupling of cortical neurons. Nat. Neurosci. 14, 217–223
- Zhang et al. 2014. Propagation of epileptiform activity can be independent of synaptic transmission, gap junctions, or diffusion and is consistent with electrical field transmission. J. Neurosci. 34.
- Qiu., R.S Schivacharan, and M. Zhang et al. 2015. Can Neural Activity Propagate by Endogenous Electrical Field? J. Neurosci. 35, 15800–11
- Zhang. et al. 2014. Propagation of epileptiform activity can be independent of synaptic transmission, gap junctions, or diffusion and is consistent with electrical field transmission. J. Neurosci. 34, 1409–1419
- C Chiang., R.S Shivacharan, and X. Wei et al. 2019. low periodic activity in the longitudinal hippocampal slice can self-propagate non-synaptically by a mechanism consistent with ephaptic coupling. J. Physiol. 597, 249–269
- S Shivacharan., C.C Chiang, and M. Zhang et al. 2019. Self-propagating, non-synaptic epileptiform activity recruits neurons by endogenous electric fields. Exp. Neurol. 317, 119–128
- Korn, and D.S Faber. 1980. Electrical field effect interactions in the vertebrate brain. Trends Neurosci. 3, 6–9
- Plonsey. 1969. Bioelectric Phenomena. McGraw Hill Series in Neural Engineering.
- Durand. 2000. The Biomedical Engineering Handbook. Electrical stimulation of excitable tissue. CRC Press snd IEEE Press
- Evgueniy V. Lubenov & Athanassios G. Siapas , Hippocampal theta socillations are travelling waves, 2009 Nature, 459:534–539
- A Schevon et al. 2012. Evidence of an inhibitory restraint of seizure activity in humans. Nat. Commun. 3, 1060
- C Chiang., X. Wei, and A. Anathakrishnan et al. 2018. Slow moving neural source in the epileptic hippocampus can mimic progression of human seizures. Sci. Rep. In Press
- T Park., G.F.B Vaca, and R. Tangen et al. 2018. Hippocampal transection for stereo-electroencephalography-proven dominant mesial temporal lobe epilepsy in a child: A detailed case report and critical review. J. Neurosurg. Pediatr. doi:10.3171/2018.5.PEDS1896
- Ntsambi-Eba., G. Vaz, and M.A Docquier et al. Patients with refractory epilepsy treated using a modified multiple subpial transection technique. Neurosurgery. doi:10.1227/NEU.0b013e31828ba750
- V Sanchez-Vives, and D.A McCormick. 2000. ellular and network mechanisms of rhythmic recurrent activity in neocortex. Nat. Neurosci. 3, 1027–1034