Dr Yu Wang, associate professor in the Department of Pharmacology and Pharmacy at the University of Hong Kong and State Key Laboratory Hong Kong, on the discovery of novel biomarkers
About Dr Yu Wang
After obtaining her PhD degree in Proteomics and Biomedical Sciences from the University of Auckland, New Zealand, Dr Wang worked as a research fellow in Maurice Wilkins Centre for Molecular Biodiscovery, and helped with the establishment of the Proteomics Research Facility. In 2004, Wang joined the Genome Research Center and the Department of Biochemistry at the University of Hong Kong. She has made a major contribution to the development of proteomics research and the establishment of various biomarker discovery platforms.
In 2008, Wang joined the Department of Pharmacology and Pharmacy at the University of Hong Kong as a faculty member. She has published seven book chapters and over 130 research articles in internationally peer-reviewed journals, with an average citation of 36.78/article, H-index 40, and obtained five US patents and one European patent. Wang is among the top 1% of highly cited scientists, according to ISI Essential Science Indicators.
Here, she speaks to Innovation News Network about her pioneering research which aims to identify potential therapeutic targets capable of slowing down and reversing the cardiovascular ageing process.
What are the main research areas?
We have been working on the field of obesity and cardiovascular diseases, and are focusing on discovering novel targets for obesity, Type 2 diabetes, and cardiovascular diseases (CVDs) associated with chronological and accelerated ageing. My team have also studied the functional characterisation of biomarkers and drug development using integrated molecular, cellular, animal and clinical approaches.
We are working on a number of other experiments to identify more promising therapeutic targets. We have already succeeded in identifying a series of novel adipokines – a group of molecules secreted from fat cells that play a central role in the regulation of energy metabolism and cardiovascular homeostasis. Specifically, we have pinpointed adipokines with both diagnostic and therapeutic values in clinical medical conditions associated with obesity, insulin resistance, hyperglycaemia, elevated blood pressure and renal diseases.
Other areas of research include identifying protein biomarkers that may prove valuable in the large-scale risk assessment, early diagnosis and therapeutic guidance of Type 2 diabetes mellitus, and other related cardiovascular and renal diseases.
What role does lipocalin-2 play in hypertension and obesity-associated medical complications?
Cardiometabolic dysfunction involves conditions of many metabolic alterations that will ultimately trigger various molecular and cellular adaptions leading to the pathological development of cardiovascular diseases. My group has been working on discovering the functions of different adipokines in the onset and development of cardiometabolic dysfunctions.
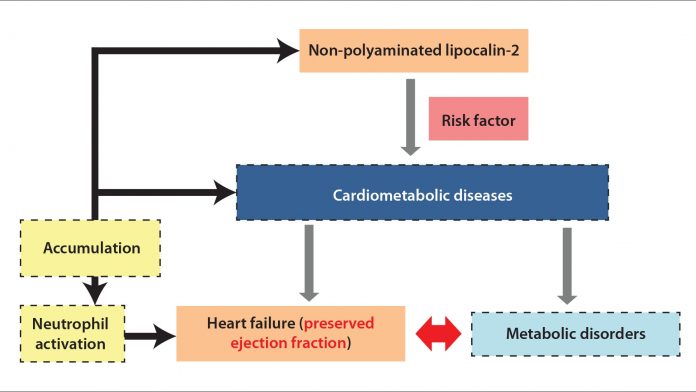
Lipocalin-2 is a typical molecule that we are working on. This protein is one of the typical molecules that may be involved in the aforementioned sequence of events. It is a pro-inflammatory adipokine which is involved in various cardiovascular diseases (including hypertension, coronary heart disease, stroke, atherosclerosis, and heart failure), and positively correlated with anthropometric metabolic variables, including insulin resistance index, hyperlipidaemia, hyperglycaemia, and inflammation. This molecule, also known as neutrophil gelatinase‐associated lipocalin, neu‐related lipocalin, uterocalin, siderocalin, or 24p3, belongs to the lipocalin family, which functions as transporters of lipophilic substances.
Our group has reported that circulating lipocalin‐2 levels are augmented in obese human subjects or those with metabolic syndrome. It can be post‐translationally modified by polyamination, which promotes the clearance of this protein from the circulation. However, the non-polyaminated form is detained in the blood and deposits in tissues such as vessels, leading to vascular dysfunction.
Our cohort studies have shown that patients with cardiac problems have higher non-polyaminated lipocalin-2 than those healthy volunteers. The levels of this form of lipocalin-2 in circulation or urine shows positive association with the risk factors of cardiometabolic disease, such as high heart rate, circulating triglycerides, high-sensitivity C-reactive protein and high creatinine levels. Accumulated non-polymination of lipocalin-2 in the pericardial fluid and pericardium of heart failure patients also indicates that lipocalin-2 is importantly involved in the development of cardiac dysfunction.
Could you briefly describe your own work in this area, particularly with regard to Endothelial Dysfunction and npLcn2 as a biomarker for cardiometabolic risk assessment?
Vascular dysfunction is a key characteristic of ageing that leads to impaired blood vessel function. Indeed, ageing is considered as an independent risk factor for decline in vascular function. Even in a healthy individual, the ageing process causes a gradual failure of the body’s innate ability to protect and preserve blood vessel integrity.
My team are working to discover more about the polyamination and non-polyamination of lipocalin-2, their roles in the development of cardiometabolic dysfunctions, and the potential to be developed as novel biomarkers of cardiometabolic diseases. Together, Dr Kangmin Yang and Andy Man, along with collaboration from Professor Jo G.R De Mey’s group at the Institute of Molecular Medicine, University of Denmark, are focused on detecting the non-polyamination of lipocalin-2 in ageing-related cardiometabolic diseases.
This work has shown that health volunteers with increasing risk factors of cardiovascular diseases, or patients with different types of heart failure, displayed distinguished profiles of distribution of non-polyaminated lipocalin-2 in different biofluids or tissues, highlighting the important role that this molecule plays in cardiometabolic diseases and, indeed, as a promising characteristic as a sensitive biomarker of cardiometabolic dysfunction.
Notably, our work is the first to report the urinary lipocalin‐2 levels in a healthy cohort of subjects and a non‐hospitalised Chinese population. My team has shown that the immunoassays used in our study are able to differentiate the pathophysiological expression of pLcn2 and npLcn2 in human samples. The levels of non-polyaminated lipocalin-2 and the ratio of different forms of the lipocalin-2 proteins could be applied to stratify obese subjects with or without metabolic syndrome, or human patients with or without a severe condition of the heart/renal failure.
What challenges have you experienced?
The post-translational modification of the lipocalin-2 by polyamination is very interesting. But how to dissect the physiological or pathological roles of the individual form of this molecule is a big challenge. It requires useful methods or assays to efficiently differentiate each form of the proteins, which should be deduced to clinical assessments.
What have you accomplished?
Different antibodies and immunoassays are developed and applied in the cohort studies. The association between different forms of lipocalin-2 and the cardiovascular risk factors is being studied. The detailed elucidation of the mechanism underlying how lipocalin-2 proteins are involved in the development of pathological events is ongoing.
What future research priorities do you have?
Going forward, my team are working on a number of other experiments to identify more promising therapeutic targets in cardio-renal metabolic diseases. With expertise in the field of immunological tools, and when developing various antibodies and immunoassays in our own and our partner’s group (see: http://www.antibody.hku.hk/), we have already been successful in identifying a series of novel adipokines – a group of molecules secreted from adipose tissues that play a pivotal role in the regulation of energy metabolism and cardiovascular homeostasis.
More specifically, our group have pinpointed adipokines, which have been shown to have both diagnostic and therapeutic values in clinical medical conditions associated with obesity, insulin resistance, hyperglycaemia, elevated blood pressure and renal diseases.
In addition to cardio-renal diseases, my team are also focusing on other research areas, including the identification of important protein biomarkers that may prove valuable in the large-scale risk assessment, early diagnosis and therapeutic guidance of Type 2 diabetes mellitus and other ageing-related cardiovascular diseases and cancers.
What benefits could your work have to both future research and treatment pathways?
Cardiovascular diseases are the leading causes of morbidity and mortality in developed countries and are becoming a major threat in developing areas now, too. It has been well recognised that metabolic disorder is the main contributor to cardiovascular diseases, which are thus collectively called cardiometabolic diseases, including stroke, coronary heart disease (CHD), myocardial infarction, atherosclerosis and heart failure.
According to the updated information on 2017 heart diseases and stroke statistics by the American Heart Association, around 92.1 million American adults are suffering from some form of cardiometabolic disease, while there is also an alarming increase of incidences amongst younger adults. Increased calorie intake, sedentary lifestyle, psychological stress and fat gain are typical characteristics of the unhealthy modern lifestyles that are contributing to the development of metabolic syndrome, including obesity, dyslipidaemia, insulin resistance, hypertension, inflammation and oxidative stress.
Cardiometabolic diseases involve not only cardiac dysfunction, but also more complicated disorders in multi-organs and their crossover, which is happening earlier than, or along with, cardiovascular problems.
Understanding the mechanisms underlying the functional and structural development, and so determining the proper way to assess the risk of cardiometabolic diseases, has been the goal and interest of many researchers for some time; these researchers have been working towards efficient prevention, diagnosis and therapeutic control of the development of cardiometabolic dysfunction.
What are your hopes for the future?
I hope to be able to foster an increased level of co-operation with different institutes, meaning that we are able to share information and knowledge, and go on to work together to discover more about cardiometabolic diseases and develop proper biomarkers for different diseases.
Dr Yu Wang
State Key Laboratory of Pharmaceutical Biotechnology
Department of Pharmacology
and Pharmacy
The University of Hong Kong
+852 39176864
yuwanghk@hku.hk
http://www.pharma.hku.hk/web2/staffweb/yuwang/index.php