More than 50 million people suffer from skin defects every year in the world, and require surgical intervention to restore skin function and appearance.
The standard of care (autografting) is ineffective and very often too scarce. Autografting is a challenge when very large body surface area is affected by the defect or the injury (donor site shortage) and it is ineffective when deep wounds are to be treated (scarring). These two unmet clinical needs may be finally solved by the bio-engineering of personalised, safe, permanent skin tissue therapy.
The clinical challenge
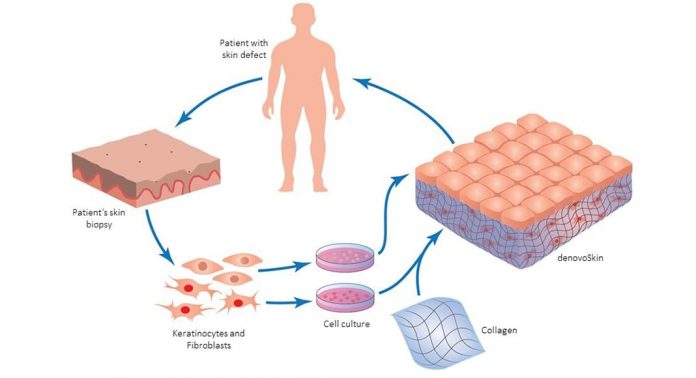
Skin cells can be isolated from the patient, expanded and combined into a skin-like graft (denovoSkin™) then transplanted on the very same patient.

Completed phase I trials have shown that the product is safe and have provided the long awaited proof of principle that denovoSkin™ can regenerate and grow once transplanted. Efficacy trials are in the recruiting phase IIb in Switzerland and the Netherlands. The indications in the Phase IIb trials are burns and reconstructive (e.g. scar revision, etc). These studies will compare denovoSkin™ to autografting on the same patients in similar body locations and graft take and scar quality will be evaluated in short and long term.
Burn compassionate patients may also represent a priceless source of data to support the clinical development of denovoSkin™. Indeed, burn compassionate patients, those with no alternative and those in deepest pain are the biggest motivation for the team at CUTISS.
The scale up challenge
Skin is the largest organ on a human body. Burn patients may need large quantities of denovoSkin™ in the shortest time possible, starting off from the smallest biopsy possible, for a price that can be reimbursed. Thus, CUTISS is working full speed on the automation of the manufacturing process. Automation is key to the success of denovoSkin™ as much as the results of the clinical trials. Indeed, the manufacturing and distribution of personalised tissue engineered therapy poses a number of business challenges that automation can greatly help to minimise.
Automation will ensure the manufacturing of large quantities of a safe product in a highly standardised manner, will reduce production time and costs, allow an easier transfer of technology to achieve a decentralised manufacturing model, and will also pave the ground of a new era in regenerative medicine. CUTISS plans to obtain the automation proof of concept in the coming months, supported by SME instrument H2020, by means of the system integration of existing technologies in combination with newly designed disposable sets.
CUTISS 2.0
CUTISS is manufacturing denovoSkin™ at the GMP facility of the Wyss Zurich, a start-up incubator of UZH and ETH in Zurich. As the next step, the company is investing in building its own GMP facility at the Biotechnopark in Schlieren and to put together a highly motivated and experienced team that can bring denovoSkin™ to the market.

This step is essential to switch gears from academia to industry settings, posing new challenges but also a brand new set of opportunities and, above all, partnerships. The team has grown from five to eighteen people and is expected to further grow in 2020. Following the successful fund raising so far, a Round B is planned to be launched latest in 2020.