The European Commission has granted marketing authorisation for Pfizer’s LITFULO (ritlecitinib) to treat adults and adolescents 12 years of age and older with severe alopecia areata.
LITFULO, an oral capsule taken once daily, is the first medicine authorised by the European Commission to treat individuals as young as 12 years of age with severe alopecia areata.
LITFULO is the first and only treatment to selectively inhibit Janus kinase 3 (JAK3) and the tyrosine kinase expressed in hepatocellular carcinoma (TEC) family of kinases.
“Today’s approval of LITFULO in Europe is an important milestone for patients as young as 12 years of age with substantial hair loss from alopecia areata, as they now have an opportunity to achieve significant hair regrowth,” said Angela Hwang, Chief Commercial Officer, President, Global Biopharmaceuticals Business, Pfizer.
“Previously, there were no treatment options approved by the EC for adolescents with severe alopecia areata, and Pfizer is proud to be bringing forward this new innovative medicine for patients living with the challenges brought by this autoimmune disease.”
The marketing authorisation for LITFULO follows a series of approvals
The marketing authorisation for LITFULO is valid in all 27 EU Member States. It is also valid in Iceland, Liechtenstein, and Norway.
The authorisation follows the recommendation for approval by the European Medicines Agency’s (EMA) Committee for Medicinal Products for Human Use (CHMP) in July 2023.
It also follows approvals from the U.S. Food and Drug Administration (FDA) and the Japanese Ministry of Health, Labour and Welfare (MHLW) in June 2023.
The approval was based on the ALLEGRO clinical trial programme
The ALLEGRO clinical trial programme, on which the approval was based, included the ALLEGRO Phase 2b/3 study (NCT03732807).
The ALLEGRO Phase 2b/3 study investigated LITFULO in patients 12 years of age and older with alopecia areata with 50% or more scalp hair loss, including patients with alopecia totalis (total scalp hair loss) and alopecia universalis (total body hair loss).
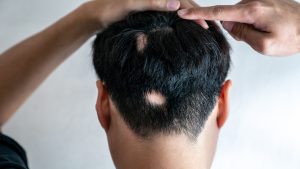
The results from the study revealed that 13.4% of adults and adolescents saw 90% or more scalp hair coverage (SALT ≤10) after 24 weeks of treatment with LITFULO 50mg compared to 1.5% with placebo.
Patient Global Impression of Change (PGI-C) response was measured and was a key secondary outcome supporting the approval.
49.2% of participants reported a response of moderate to great improvement in their alopecia areata at week 24, compared to 9.2% with placebo.
About ALLEGRO-LT
ALLEGRO-LT (NCT04006457) is an ongoing Phase 3, open-label, long-term study, with safety and efficacy data being collected for adults with alopecia areata with 25% or greater scalp hair loss. The study also includes adolescents from 12 years of age with alopecia areata with 50% or greater scalp hair loss.
The approval was also supported by the long-term efficacy and safety data from this study.
The most common adverse reactions reported with LITFULO included diarrhoea (9.2%), acne (6.2%), upper respiratory tract infections (6.2%), urticaria (4.6%), rash (3.8%), folliculitis (3.1%), and dizziness (2.3%).









