Ming Tang, Associate Professor in the Department of Materials Science & Engineering at Clemson University, details how the innovative phosphate-based waste form platform can remedy advanced reactor nuclear waste.
Nuclear power produces a significant percentage of energy within the United States. At the same time, the nuclear industry generates waste during energy production (electricity). However, some of these nuclear wastes are highly radioactive and must be isolated durably from the biosphere in hard-wearing matrices for several thousands of years or more to limit their potential impact on the environment. Nuclear waste forms are at the centre of a successful strategy for the clean-up and isolation of radioactive waste from the environment. The term waste form is defined by the International Atomic Energy Agency (2003) as waste in its physical and chemical form after treatment and/or conditioning (resulting in a solid product) prior to packaging. The radioactivity is entirely contained in the waste form, which is the first barrier to the release of radionuclides, making an important contribution to the performance of the disposal system.
The history of nuclear waste form development and evaluation spans over more than 40 years. During that time, there have been new ideas about the types of materials that could be used; innovations in the technologies to produce these materials; new strategies for evaluating their performance in a geologic repository; and substantial advances in the relevant fields of materials science, geochemistry, processing technologies, and computational simulations. This report focuses on the phosphate-based waste forms for treating advanced reactor (AR) waste, specifically molten salt reactor (MSR) applications.
The MSR is one of the leading advanced nuclear reactor candidates to replace current nuclear reactor technologies in the US. In general, two main categories of MSRs are those cooled with molten salts (salt-cooled reactors) and those fuelled and cooled by molten salts (salt-fuelled/cooled reactors). For MSR operation, salt treatments can mitigate fission product accumulation in the electrorefiner or MSR core, but eventually, the salt will need to be replaced if neutron poisons exceed a concentration threshold.
Electrochemical reprocessing, also called pyroprocessing, is one option to reprocess used nuclear fuel for recycling used UO2 and mixed oxides in advanced nuclear cycles. During electrochemical reprocessing, used fuel is dissolved in molten salt (e.g., LiCl-KCl eutectic), while the salt stream is the used salt in the electrorefiner containing dissolved fission products. Significant quantities of salt-laden waste streams are generated during MSR operations and subsequent electrochemical reprocessing/pyroprocessing. The greatest challenge in safely disposing of the MSR and electrochemical reprocessing waste streams is the high concentration of water-soluble salt phases, which must be immobilised in a chemically durable waste form. The major components of typical salt waste streams are chloride-based and/or fluoride-based salts, rich in alkali halides and/or alkaline earth halides. Fission products and actinides, which may be recovered, are also contained in salt waste streams.
Common waste form options for salt-based high-level waste (HLW) include glasses, ceramics, glass ceramics, or glass-bonded ceramics (alternatively called glass-composite waste forms). Up until now, two approaches to the immobilisation of salt wastes have been suggested. One is direct immobilisation using Cl and/or F-containing waste forms. However, waste form options are significantly limited if the halides are not removed beforehand. Due to the low solubilities (< 1.5 mass % for silicate-based glass) of the chloride/fluoride ions and the evolution of Cl2/F2 gas from the melt under operation temperature, it is not suitable to employ the borosilicate and iron phosphate glasses as the host for the full-salt waste streams.1 Of the minerals incorporating Cl, potentially viable systems include zeolite-based and calcium phosphate-based phases. Zeolites and sodalites/glass-bonded sodalites have been extensively studied in the US as candidates for immobilising chloride pyrochemical wastes generated during the reprocessing of fuel from experimental breeder reactor programmes.2 Waste loading in these waste forms is limited by stoichiometry to form and encapsulate chloride-bearing sodalite (~ 8 mass %), at which point residual salts are observed.2
Phosphates as nuclear waste forms are not a new concept. A significant effort has been made by the scientific community to evaluate the potential of phosphate minerals (e.g., monazite) and glasses as nuclear waste storage hosts since the 1970s.1 The great advantage of these materials as waste storage hosts is that they can incorporate substantial quantities of actinides in their structure and are chemically stable. However, only calcium phosphate was explored for the immobilisation of salt waste streams.
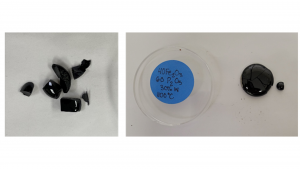
Phosphate glass
In early studies,3,4 phosphate-based glass waste forms had been developed for HLW generated by reprocessing spent nuclear fuel, particularly for actinides. Then adding lead and iron oxide to a phosphate glass was found to substantially improve the chemical durability to the levels required for waste vitrification. Further, the waste loading can be increased to more than 35 wt % in iron phosphate glass, and chemical durability was not inferior to borosilicate and other common glasses. Iron-phosphate glass waste forms (Fig. 1)5 are being developed under the auspices of the U.S. Department of Energy (DOE), which can be used to immobilise salt wastes from the electrochemical reprocessing of used nuclear fuel. These waste forms are appropriate for oxide-fuel or metal-fuel (electro) reduction (OR) and electrorefining (ER) waste salts at intermediate melting temperatures (950 – 1,200 °C).6 Previous studies demonstrated that phosphate glasses had dissolution rates 100 to 1,000 times lower than those of borosilicate glass.7

Other phosphate glasses, specifically stannous fluoro-phosphate (Sn-P-O-F) glass, were reported to possess relatively low glass transformation temperatures, acceptable chemical durability, good water resistance, and comparable to those of commercial soda-lime silicate glasses.7 SnF2-P2O5 glass waste forms (Fig. 2) with low melting temperature (400-600 °C) and loaded up to 50 wt % salt waste streams have been reported recently.5 Chlorophosphate glasses in the systems Sn–Pb–P–O–Cl and Sn–O–P–Cl have been developed by replacing fluorine with chlorine. The range of melting temperature in these glasses systems is between 400-600°C or even lower, which could avoid the volatilisation of chloride and fluoride and improve salt retention or solubility. It suggests that these glasses may be suitable for immobilising chloride-based and/or fluoride-based salt streams through vitrification.
Phosphate apatite
Apatite is the most abundant naturally occurring phosphate on Earth. The general formula: M10(XO4)6Z2, where M is a variety of +1 to +3 charged cations (e.g., Ag, K, Na, Cs, Ca, Ba, Pb, Sr, La, Ce), X is generally a tetrahedral cation (e.g., Mn5+, As5+, Cr5+, V5+, P5+, Mo5+, Si4+, Ge4+, Re7+, and Tc7+), and Z is OH–, F–, Cl–, I–, are extremely diverse in composition. Phosphate apatite minerals corresponding to the general formula M10(PO4)6(Z)2, referring to hydroxyapatite, fluorapatite (FAP), and chlorapatite (ClAp) have been investigated as potential nuclear waste forms to incorporate rare earth elements and actinides, as well as a host phase for waste chloride salt from pyroprocessing of spent nuclear fuel.8 Specifically, successfully fabricated dense fluorapatite Ca10-2xLaxNax(PO4)6F2 (x=0.2 and 2) and fluoridated chlorapatite Ca10(PO4)6FxCl2-x (x=0, 0.5, 1, 1.5, 2), demonstrated that phosphate-based apatite ceramics are capable of retaining chloride and fluoride in stable mineral phases with/without rare earth and alkali ions, and furthermore that the structure has multiple crystallographic positions available to accommodate a large array of diverse elements.8 A number of prior data indicate apatite as considerably more durable than sodalite, particularly when exposed to water. Apatite dissolution is orders of magnitude slower than the dissolution of borosilicate glass in an acid environment.8
The composition design of single-phase phosphate apatite M10(PO4)6(Z)2 will be alkali, alkaline earth, and rare earth (RE) ions that go to the M position and halogens that go to Z position as tunnel ions. Regarding the waste loading, a maximum theoretical chlorapatite loading of 10.5 mass % (6.2% Cl) is possible. Additional chloride, up to a total of 26 mass % (16.8% Cl), may be accommodated through the further reaction of CaCl2 with chlorapatite to form spodiosite Ca2 (PO4) Cl. Specific apatite, such as Pb-V-I or Ca-P-I systems, focus on iodine immobilisation. Other systems could incorporate other radionuclides such as Tc, Cs, and Sr. Phosphate apatite phases with various compositions could be applied to incorporate multiple waste streams and increase waste loading.
Impact of nuclear waste forms
Several factors must be considered when developing waste form material for immobilising a specific waste stream. The key considerations include the following: high waste loading to minimise the volume and the space needed for disposal; low rate of dissolution to minimise the release of radioactive and chemical constituents; ease of production under reasonable conditions like low temperatures and in an air atmosphere to minimise worker dose and the capital cost of the plant; chemical flexibility to accommodate radioactive and chemical constituents; the availability of natural mineral or glass analogues about the long-term performance in the natural environment; compatibility with the intended disposal environment.
According to the previous experimental and computation studies on apatite phase and phosphate glass, there is a significant amount of information from which to design chemically durable phosphate-based and/or apatite-based waste forms for the immobilisation of salt waste streams, fission products, actinides, and other radionuclides. The available information and knowledge provide a solid base to transform these scientific discoveries into this waste form platform which aims to develop innovative phosphate glass waste forms with low melting temperature and high salt waste loading, and various types of apatite waste forms for the immobilisation of different types of waste stream using different reprocessing technologies.
Apatite-based crystalline phases are significantly different from the traditional Synroc (synthetic rock) type waste forms which are composed of titanate minerals, e.g. hollandite, zirconolite and perovskite, etc. More importantly, although the various types of Synroc-type waste forms have been investigated since 1978,9,10 none are compatible with salt waste streams. Phosphate glass (Sn-P-O-F) in Fig. 2 possesses a lower melting temperature (400°Cs) and higher waste loading (at least 50 wt%) than current iron phosphate glass waste forms (above 1,000°C processing temperature, and up to 40 wt% waste loading). Higher waste loading implies not only smaller total volumes but also smaller unit volumes. Smaller volumes are also easier to handle and dispose of in boreholes with restricted diameters. Lower processing temperature means less operational risk, less complications, and fewer staff to maintain safety levels. These will reduce transportation, disposal, and operational costs directly.
No single waste form is suitable for all nuclear waste streams or for all disposal environments. A ‘toolbox’ of waste forms is needed for different waste streams and disposal environments. The phosphate-based waste form platform is intended to provide information to support improvements in methods for processing waste and selecting and fabricating waste forms.
References
- B J Riley, et al., “Dehalogenation of electrochemical processing salt simulants with ammonium phosphates and immobilisation of salt cations in an iron phosphate glass matrix”, Journal of Nuclear Materials, 529: 151949 (2019)
- B J Riley, et al., “Glass binder development for a glass-bonded sodalite ceramic waste form”, Journal of Nuclear Materials, 489: 42-63 (2017)
- B C Sales and L A Boatner, “Lead phosphate glass as a stable medium for the immobilisation and disposal of high-level nuclear waste”, Materials Letters 2 (4, Part 2):301-304 (1984)
- B C Sales and L A Boatner, “Lead-Iron Phosphate Glass: A Stable Storage Medium for High-Level Nuclear Waste”, Science, 226 (4670): 45-48 (1984)
- M Tang, “Development of Phosphate Glass Waste Forms To Immobilise Salt Waste Stream”, Glass and Optical Materials Division Annual Meeting, May 22-26, 2022, Baltimore, Maryland, US
- W L Ebert, et al., “Durability Testing of Developmental Iron Phosphate Waste Forms for Echem Salt Waste”, NTRD-MRWFD-2018-000513 (2018), Argonne National Laboratory
- E H Oelkers, E Valsami-Jones, “Phosphate mineral reactivity and global sustainability”, Elements 4:83– 87.Elements, 4:113-116 (2008)
- C Ye, et al., “Heavy Kr ion irradiation-induced amorphisation on fluorapatite Ca10-2xLaxNax(PO4) 6F2(x=0.2 and 2)waste form with varying La loading”, Journal of Alloys and Compounds, 822: 153756 (2020)
- D J Gregg, et al., “Synroc technology: Perspectives and current status (Review)”, Journal of the American Ceramic Society, 103:5424-5441 (2020)
- E R Vance, et al., “Candidate waste forms for immobilisation of waste chloride salt from pyroprocessing of spent nuclear fuel”, Journal of Nuclear Materials, 420: 396-404 (2012)
Please note, this article will also appear in the twelfth edition of our quarterly publication.









