The mechanism of photoionisation has been used to highlight how complex pathways influence time delays in the ionisation of molecules.
How can researchers employ the mechanism of photoionisation to gain an insight into complex molecular potential? This question has now been answered by a research team, led by Professor Dr Giuseppe Sansone, from the Institute of Physics at the University of Freiburg.
The research team comprises of scientists from Freiburg, the Max Planck Institute for Nuclear Physics in Heidelberg, the Universidad Autonoma in Madrid, and the University of Trieste. Their results have been published in the journal Nature Communications.
Photoionisation process
In the origin of photoionisation, also named the photoelectric effect, an atom or molecule absorbs one quantum of light, usually indicated as photon, from an external field. The energy absorbed in this process is transferred to an electron, which is liberated, leaving behind a singly charged ion.
In several aspects and for several applications, the impact can be regarded as instantaneous, meaning that there is no significant time delay between the absorption of the photon, and the instant when the electron is emitted.
However, several experiments conducted in the last couple of years have evidenced that tiny, yet measurable delays lying in the attosecond range (1 as=10-18 s), occur between these two processes.
Generation of attosecond pulses
“Thanks to the advanced laser sources and specially designed spectrometers available in our laboratory, we can generate the shortest bursts of light, lasting only few hundreds of attoseconds,” Sansone explained. “Moreover, we can reconstruct the orientation of simple molecules when they absorb a photon from an external laser pulse. We have used such pulses to investigate the motion of the electrons after the absorption of a photon.”
Electrons experience paths with potential peaks and valleys
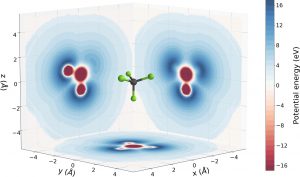
The researchers discovered that on its way out from the molecule, the electron experiences a complex landscape characterised by potential peaks and valleys. These are determined by the spatial distribution of the atoms composing the system. The path followed by the electron during its motion can impact the time it takes to be freed.
Extension to more complex molecular systems made possible
In the experiment, the team measured the time delays accumulated by the electrons emitted from CF4 molecules in different spatial directions, which were measured utilising an attosecond pulse train combined with an ultrashort infrared field.
“Combining this information with the characterisation of the spatial orientation of the molecule, we can understand how the potential landscape and, in particular, potential peaks affect the time delay,” concluded Sansone.
The work can be extended to more complex molecular systems and to potentials changing on ultrashort timescales. In general, Sansone emphasises, this approach could give the possibility to map complex potential landscapes from within, with unprecedented temporal resolution.









