Integrating the concepts of Radioactive Waste Management with Life Cycle Analysis towards a circular economy.
Radioactive waste is a by-product from nuclear reactors, fuel processing plants, hospitals, and research facilities. It is also generated while decommissioning and dismantling nuclear reactors and other nuclear facilities. Radioactive waste comes from many sources, including nuclear medicine, nuclear research, nuclear power generation, nuclear decommissioning, rare earth mining, and nuclear weapons reprocessing.1,2
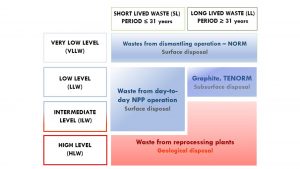
In France, the classification is done based on two parameters:3
1) The level of radioactivity:
The level of radioactivity in radioactive waste is usually expressed in Becquerel (Bq) per gram or kilogram. There are four different activity levels for radioactive waste: high-level activity (HA), mid-level activity (MA), low-level activity (FA), and very low-level activity (TFA). Very-low-level activity waste ranges from 0 to 100 Bq/g, and the subsequent categories have initial activities increasing by (typically) three orders of magnitude for each of the categories. Thus, low-level wastes range from 100-100,000 Bq/g, intermediate-level wastes range from 100,000-100,000,000 Bq/g, and high-level wastes exceed 108 Bq/g.1,3
2) The half-life:
Half-life, expressed in years, days, minutes or seconds, quantifies the time at which the initial activity of a radionuclide is halved. The half-life threshold is defined by the half-life of Cs-137 and is thus set at 31 years. Waste is distinguished by which main radionuclides have a short period (less than or equal to 31 years) and those of a long period (greater than 31 years). For the former, it is generally considered that the radioactivity is greatly attenuated after ten periods, i.e. about 300 years.
Radioactive metallic waste refers to radioactive waste that contains metal components. During decommissioning of nuclear power plants (NPPs), mainly two types of radioactive metal waste are generated, such as volumetric radioactivity induced by neutron activation of certain elements in reactor components such as reactor pressure vessel (RPV) and adjacent structure; and surface radioactivity deposited on surfaces of various out-of-core components.4 A significant fraction of the metallic wastes are out-of-core reactor components, such as heat transport pipes and heat exchanger tubes. Different types of metals are used in the construction of NPPs, including stainless steels (SS), carbon steels, and Ni-alloys.
Metals can get contaminated in a nuclear reactor through various ways, such as corrosion, radiation damage, and neutron activation. Corrosion in nuclear reactors can occur due to exposure of metals to high temperature, pressure, and corrosive chemicals in the reactor environment.5 Corrosion Products (CP) are metallic elements (cobalt, iron, nickel, etc.) directly released under an activated form or which are activated when passing under neutron flux. The main CP responsible for dose rates are 60Co and 58Co, the others are 51Cr, 54Mn, 59Fe.6,7 These products lie in the corrosion layers of the metals, and the decontamination of these metals generally involves the removal of the corrosion layers on the surface of the metal with minimal attack of the base metal.
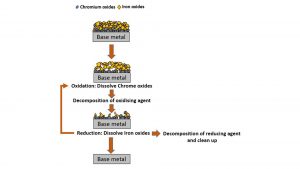
There are several methods for decontaminating radioactive waste metals, such as chemical reagent decontamination, laser decontamination, and electrocoagulation.8 One such decontamination method using chemical reagents is the Chemical Oxidation Reduction Decontamination (CORD) process, which is a worldwide-known, two-step decontamination process.9-11 In the first step, permanganate ion (MnO4-) is used to oxidise the chromium oxide-layer and to release chromate ions.9,12 In the next step, oxalic acid is added to reduce the permanganate ion to aqueous Mn2+ ions and to dissolve the Fe and Ni-enriched oxide layer from the surface of the alloys.9,12 The whole process is presented in Fig. 2, with each step lasting between three to six hours and can be repeated several times.
The solutions can then be treated with ion exchange resin to extract the dissolved metals14 or can undergo a precipitation process to precipitate the metals and reduce the use of resins, further reducing radioactive waste,9 whereas the remaining oxalic acid is destroyed by the addition of H2O2 in the presence of UV/heating.
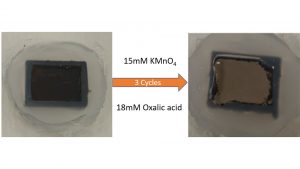
Fig. 3 shows the application of the optimised CORD process on a surrogate SS-316 sample. It is seen that it takes three cycles of the CORD process, with each step of three hours, to completely remove the oxide layer on the surface of the exposed metal.9
Decontamination activities tend to generate large volumes of radioactive waste, liquid waste in the CORD process, and therefore aligning the concepts of Life Cycle Analysis (LCA) with decontamination would promote a sustainable solution. LCA is a method used to evaluate the environmental impact of a product through its life cycle, encompassing extraction and processing of the raw materials, manufacturing, distribution, use, recycling, and final disposal.15,16
Note: This work was carried out under the PREDIS European project. This project has received funding from the European Union’s Horizon 2020 research and innovation programme under agreement No 945098.
References
- “The Geological Society of London – Geological Disposal of Radioactive Waste.” www.geolsoc.org.uk (accessed Mar. 12, 2020)
- USNRC, “Backgrounder on Radioactive Waste.” www.nrc.gov (accessed Apr. 04, 2023)
- ANDRA, “Waste classification.” www.international-andra.fr (accessed Apr. 04, 2023)
- W. H. Lee and J. H. Cheong, “Potential radiological hazard and options to cope with consequences from recycling of activated metal waste disposed of at a near-surface disposal facility,” Ann. Nucl. Energy, vol. 152, p. 107993, Mar. 2021, doi: 10.1016/j.anucene.2020.107993
- T. Do et al., “Methodology for volume reduction of radioactive metallic waste,” Radwaste Solutions. https://www.ans.org (accessed Apr. 04, 2023)
- T. F. J. Marchl, Occupational radiation exposures in Siemens designed PWRs. France: Societe Francaise d’Energie Nucleaire., 1994
- B. C. Friedrich, “Reduction of cobalt 60 inventory in light water reactors,” Kerntechnik, vol. 54, no. 2, pp. 109–113
- L. Zhong, J. Lei, J. Deng, Z. Lei, L. Lei, and X. Xu, “Existing and potential decontamination methods for radioactively contaminated metals-A Review,” Prog. Nucl. Energy, vol. 139, p. 103854, Sep. 2021, doi: 10.1016/j.pnucene.2021.103854
- A. Rivonkar et al., “Optimisation of the chemical oxidation reduction process (CORD) on surrogate stainless steel in regards to its efficiency and secondary wastes,” Front. Nucl. Eng., vol. 1, 2022, doi: 10.3389/fnuen.2022.1080954
- 10. K. Radó et al., “A systematic study of the corrosion effects of the FRAMATOME CORD-UV technology,” Acad. Appl. Res. Mil. Sci., vol. 3, pp. 171–175, 2004
- H. Wille, H. O. Bertholdt, and F. Roumiguiere, “Chemical decontamination with the CORD UV process: principle and field experience,” presented at the Fourth Regional Meeting: Nuclear Energy in Central Europe, Slovenia, Slovenia, 1997
- Z. Tian, L. Song, and X. Li, “Effect of Oxidizing Decontamination Process on Corrosion Property of 304L Stainless Steel,” Int. J. Corros., vol. 2019, pp. 1–6, Aug. 2019, doi: 10.1155/2019/1206098
- Decommissioning and Radioactive Waste Processing: Hitachi-GE Nuclear Energy, Ltd.” https://www.hitachi-hgne.co.jp/en/activities/waste_treatment/index.html (accessed Apr. 05, 2023)
- D. O. Campbell, D. D. Lee, and T. A. Dillow, “Ion-exchange processes for low-level liquid waste treatment,” in Proceedings of the international meeting on nuclear and hazardous waste management, United States, 1990. doi: 10.2172/6036184
- “Life Cycle Analysis – an overview | ScienceDirect Topics.” https://www.sciencedirect.com/topics/earth-and-planetary-sciences/life-cycle-analysis (accessed Apr. 05, 2023)
- US EPA, “Life Cycle Assessment (LCA), Sustainable Technology Research.” http://www.epa.gov/nrmrl/std/lca/lca.html (accessed Apr. 05, 2023)
Please note, this article will also appear in the fourteenth edition of our quarterly publication.