A team of scientists have identified a method for removing harmful micropollutants from the environment by employing a novel chemistry approach.
The discovery, achieved by US Army-funded research at Cornell University, has developed an innovative chemistry technique that can potentially revolutionise strategies aimed at eradicating micropollutants – biological or chemical contaminants that have detrimental effects on the planet by entering the ground and surface waters.
The research is published in Nature Communications.
Combatting micropollutants with molecules
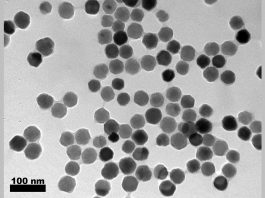
The team employed a pioneering imaging technique to attain high-resolution images of how ligands – molecules that attach to other metals or molecules – interact with the surface of nanoparticles, which led them to a groundbreaking discovery. The researchers found that altering the concentration of an individual ligand can exploit the shape of the particle it is attached to, potentially creating a strategy to remove micropollutants. This could be utilised for a plethora of daily applications, such as developing chemical sensors that are sensitive at an extremely low level to a specific chemical in the environment, enabling scientists to target particular micropollutants.
“Professor Peng Chen’s work allows for deep insights into molecular adsorption processes, which is important to understand for designing molecular sensors, catalysts, and schemes to clean up micropollutants in the environment,” said Dr James Parker, programme manager of the US Army Research Laboratory. “This research is also important for designing and engineering stimuli-responsive materials with a specialised function that could not be found in regular, bulk materials.”
The investigation analysed the interactions of ligands, obtaining a comprehensive understanding of their strength and affinity of absorption as well as their behaviour in groups.
Professor Peng Chen, the leader of the research from Cornell University, said: “When the molecule adsorbs on the surface of a nanoscale material, it also actually protects the surface and makes it more stable. This can be utilised to control how nanoscale particles grow and become their eventual shape. And we found we can do this with just one ligand. You don’t do any other trick. You just decrease the concentration or increase the concentration, and you can change the shape.”
Inventing a new imaging technique
Examining the interactions between ligands and nanoparticles was a particularly testing aspect of the micropollutants study, as absorbed ligands are hard to identify as other molecules are present in the mix, with nanoparticle surfaces also being uneven and multifaceted, requiring extremely high spatial resolution to be observed.
The surface structure and size of nanoparticles are tied to the particle’s potential applications, meaning the larger the particle, the more atoms can fit inside it. In contrast, smaller particles have less internal space but more surface volume ratio for particles to sit atop, where they can effectively be used as catalysts or for absorption. The differing structures that the atoms and molecules form on these surface facets directly correlate to the shape of the particles.
Until now, accomplishing nanometre resolution of these particles has been unsuccessful due to lacking the proper imaging method, meaning the intricacies of the various surface facets and properties of ligands could not be explored. Therefore, the researchers developed their own imaging technique called COMPetition Enabled Imaging Technique with Super-Resolution (COMPEITS) to overcome this obstacle.
The neoteric method works by introducing a molecule that reacts with the surface of the particle, producing a fluorescent reaction. Subsequently, a nonfluorescent molecule is sent to bind to the surface, where the reaction competes with the fluorescent signal. The resulting decrease in fluorescence creates a negative image that can be mapped and measured using super high resolution.
The team employed COMPEITS on a gold nanoparticle from which they were able to calculate the strength of ligand absorption, discovering that they have diverse behaviour, sometimes aiding each other in absorption and at other times hindering each other’s performance with this sometimes co-occurring.
Furthermore, the researchers discovered that the surface density of absorbed ligands decides which facet is dominant, inspiring the team to alter the concentrations of individual ligands to modify the particle’s shape entirely.
Chen said: “For us, this has opened more possibilities. For example, one way to remove micropollutants, such as pesticides, from the environment is to adsorb micro-portions on the surface of some adsorbent particle. After it is adsorbed on the surface of the particle, if the particle is a catalyst, it can catalyse the destruction of the micropollutants.”