International Editor Clifford Holt spoke to the University of Huddersfield’s Professor Alan Smith about a newly-developed method of using gel particles as a suspending media for 3D bioprinting replacement soft body tissues.
Using 3D bioprinters to produce replacement human tissue is one of the most exciting developments in medical science, but it is not without its challenges, not least when attempting to use bioprinters to replicate the softer tissue layers due to the fact that the polymers typically used have an exceptionally low viscosity when they are in a liquid state.
In an attempt to overcome such challenges, Professor Alan Smith, Director of The Biopolymer Research Centre at the University of Huddersfield, and his fellow researchers have made a breakthrough that could expand the scope and use of the technology.
The Innovation Platform’s International Editor, Clifford Holt, spoke to Professor Smith about their newly-developed method of using gel particles as a suspending media and its potential applications in 3D bioprinting.
Could you begin by outlining what you feel have been the biggest advances in recent years with regards to 3D bioprinting? And what have the major limitations been when it comes to replicating the softer layers found in human tissue?
There have been some fantastic advances in 3D bioprinting recently in terms of both the printing technology and the complexity of tissues being bioprinted. For example, breakthroughs in printing technologies have facilitated the development and installation of a 3D bioprinting facility on board the International Space Station. Back on Earth, there has also been some exciting proof of concept studies where researchers have bioprinted a range of quite complex tissue types. Particular highlights include parts of the cornea, functional ovarian follicles, and even bioprinting vascularised tissues such as heart and lungs have seen some recent progress, although these complex tissues are still some way off being ready for human clinical trials.
One of the major limitations at the moment in 3D bioprinting soft tissue structures is the lack of available ‘bioinks’ that are suitable for the bioprinting process. Generally, a bioink needs to have all of the following properties:
- They must have a mechanical behaviour similar to living tissue;
- The need to be biologically compatible with the cells loaded into the bioink and the physiological environment when implanted;
- They must not degrade into toxic compounds;
- They need to support the growth of cells loaded into the ink; and
- Importantly, they need to change from a liquid to a solid rapidly on demand.
Using biopolymers similar to those found in extracellular matrix as the scaffold material in the bioink is a logical starting point to ensure biocompatibility and to achieve a similar mechanical behaviour in the printed construct to that of living tissue. These materials, however, are not so conducive to the 3D bioprinting process. This is because the individual biopolymer components of extracellular matrix such as collagen and glycosaminoglycan, when in the in the liquid state, tend to be of very low viscosity and have relatively slow gelation kinetics when converting from a liquid to a solid structure following deposition. Therefore, when using standard extrusion bioprinting, the low viscosity liquid bioink can spread quickly when deposited onto a planar surface before crosslinking can be initiated, thereby resulting in poor resolution at best, or even completely misshapen and collapsed structures.
To overcome these issues, high viscosity biopolymers are often used as they prevent the collapse of the structure prior to gelation. This can, however, require increased extrusion pressures to deposit the material, subsequently reducing cell viability and can also impact on cell phenotype when incorporating cells that require softer substrates.
Another major issue is creating the variations in microstructure that replicates that of complex tissue throughout the printed part. A particular problem with many biopolymer materials is that once a deposited layer is fully solidified it can be difficult to integrate the subsequent layers. This also causes difficulties if variations in physical, chemical, or biological behaviour is required in different sections of a single structure, which could potentially be achieved by using a different bioink for specified regions. However, it is difficult to passively adhere disparate biopolymers materials layer by layer once solidified as they tend to simply slide off one another. This prevents the production of large, geometrically complex constructs that are anisotropic or that contain gradients of mechanical behaviour, differing in chemistry or containing different cell types. This is a critical issue to overcome as it is this complex architecture that allows native tissue to perform its function.
How does the method of using gel particles as a suspending media that you have developed help to overcome these challenges? What was involved in the research? What hurdles did you encounter when working to develop the gel?
To overcome these challenges, we have developed a technique called suspended layer additive manufacture (SLAM) which uses the self-healing and viscoelastic properties of a ‘fluid gel’ as a suspending medium to prevent collapse of the low viscosity bioinks when deposited. Using the suspending medium allows the liquid bioink to be deposited and remain in a liquid state without changing shape. The printed liquid can then be solidified accordingly once printing is complete. This not only overcomes problems associated with maintaining shape fidelity but also makes it possible to integrate alternative bioinks with different properties to separate parts of the bioprinted structure, creating regional variations in physical chemical and biological properties within a single construct that are more akin to native tissue.
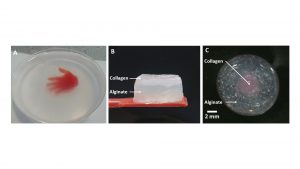
When developing this technology, we needed to find or develop a material with very specific properties. The gel needed to be shear thinning to facilitate the depositing needle of the bioprinter to move freely through the material. But at the same time, it needed to restructure quickly in order to support the deposited bioink. Moreover, it was important that the gel material would not integrate with the deposited bioink and that once the printed structure was solidified and ready to be removed from the suspending media the gel could be easily washed off without damaging the structure. Another desired property was for the gel to be stable at 37°C so that, if needed, the 3D bioprinted structure could be incubated in situ within the suspending media until required.
We managed to achieve all of these properties using an agarose particulate fluid gel. Fluid gels (sometimes termed ‘sheared gels’) can be prepared from many gel-forming polymers by applying shear as the material passes through its sol-gel transition. The resulting material is a suspension of gel particles that can be poured like a liquid but have solid-like behaviour when at rest, which is in stark contrast to the solid bulk gel that would be produced if allowed to gel quiescently.
Furthermore, these materials are created through a mechanism similar to that of crystallisation and growth, which therefore gives rise to various microstructures that include spherical particles, ellipsoids, elongated structures, and hairy particles. This is distinctly different to the gelled particles that are obtained by mechanically breaking a solid bulk gel into a slurry.
Importantly, the nature of the fluid gel microstructure produced can be controlled during manufacture and it depends on several environmental parameters such as shear rate, temperature, and those that are intrinsic to the material such as chemical structure and gelation kinetics. When processing agarose in a specific way, it was possible to generate a fluid gel consisting of microscopic hairy particles that entangle at rest, creating solid like behaviour. However, with the addition of a small amount of agitation, they can easily flow and, importantly, once the agitation force has been removed, the particles quickly re-entangle reverting to a solid-like behaviour. This behaviour was deemed ideal for suspending medium.
In addition, agarose gels have many other favourable properties, including thermal stability at 37°C, they are biologically compatible, they are from a non-animal origin, are non-toxic, are transparent, and can easily be washed off the printed construct.
Although it is relatively easy to make an agarose fluid gel, we found that any small deviations in the parameters used during manufacturing can result in different particle sizes, and subsequently, a change in the performance of the fluid gel as a suspending medium. As such, optimising the performance and reproducibility of the fluid gel was initially a significant challenge that was overcome by carefully controlling production conditions.
What will the main applications of the gel be (osteochondral plugs to repair cartilage defects etc.)?
The main function of the fluid gel material in bioprinting will be to allow printing of low viscosity bioinks, which will enable the printing of various soft tissues and also facilitate the printing of tissue interfaces between hard and soft tissue. Thus, replicating tissues with natural gradients such as osteochondral region, skin, or neural interfaces.
We believe there are, however, many additional applications that we are only just beginning to realise. These include developing disease models, developing tissue replicas as in vitro drug screening platforms, and in developing more sophisticated wound dressings.
There are also wider applications of fluid gel materials beyond bioprinting due to their tuneable multifunctional material behaviour. We have recently explored using fluid gels as drug delivery systems, for instance, and have developed modified release oral liquid medicines, mucoadhesive nasal sprays, and topical gel formulations, while other groups have investigated using these materials to deliver bioactives in ophthalmic formulations and in cell delivery.
How important is it for the new manufacturing process to allow for the use of a wide range of polymeric materials, including many already approved by regulatory bodies? Are there any yet to be approved materials that you also feel could have added benefits?
Formulation of a range of bioinks is paramount for the successful development of bioprinted living structures. This technology will facilitate the use of materials that are not compatible with conventional printing techniques. Indeed, we have demonstrated that it is possible to 3D print with bioinks that have viscosities as low as water. We believe this will enable the use of more materials, in particular biopolymers, many of which already have regulatory approval but are currently poorly compatible for bioprinting systems either due to low viscosity or lengthy gelation kinetics. This will subsequently result in a quicker route to clinical trials of bioprinted constructs.
It is important to note that there are other fluid gels or even gel slurries (mechanically homogenised gels) that can be used as suspending medium for 3D bioprinting that have their own particular advantages and disadvantages depending on the type of tissue to be printed, the bioinks used, and material used to create the suspending medium.

Moving forwards, you also plan to investigate the integration of stem cells into the system. What do you hope to achieve here, and what are your more general hopes for the future?
We have recently undertaken work with adipose derived mesenchymal stem cells and demonstrated that they can be differentiated following bioprinting using SLAM. This has enabled us to 3D bioprint a full thickness skin model (epidermis, dermis, and hypodermis) containing adipocytes that had differentiated from the loaded stem cells within the hypodermal layer.
We are currently exploring the use of SLAM to create a range of soft tissue models that include skin to assess topical drug delivery and as potential wound healing solutions, and brain cancer models to test potential drug treatments.
My hope for the future of this system is for it to be used as an enabling technology to create a bioprinted implant that is functional and ultimately improves the quality of life of the recipient.
Professor Alan Smith
Director, The Biopolymer Research Centre
University of Huddersfield
a.m.smith@hud.ac.uk
Tweet @profalanmsmith @HuddersfieldUni
https://research.hud.ac.uk/institutes-centres/centres/biopolymerresearchcentre/
Please note, this article will also appear in the seventh edition of our quarterly publication.









