Revivo Biosystems’ organ-on-a-chip platforms drastically increases human relevance and productivity and enables future targeted developments for the chemical, personal care and pharmaceutical industries, transforming in vitro research.
Prior to market launch of personal care products and the clinical trials of therapeutics, stakeholders within the chemical, ingredient, FMCG, and pharmaceutical industries need to assess and determine their safety, absorption and efficacy. Traditionally, these evaluations have been conducted in vivo on animal models, resulting in more than 100 million animals used and killed in lab tests every year.1 These figures are even more shocking when it is considered that animal models are often inaccurate and suffer from failure rates as high as 90%. Obvious genetic and biological differences exist between the test animals and the eventual human recipients.
Conventional non-animal alternative methods rely on ex vivo human tissue explants and in vitro tissue engineered models. Though these methodologies have evolved over the last two decades, they still lack critical functions to realistically resemble human tissues and are unable to consistently deliver results accurately and reproducibly. Current approaches rely on static culture processes and tedious manual operations; hence, they are not only unable to recapitulate the complexity of human tissue’s structure and microphysiological environment, but they also fail to enable a substantial reduction in R&D expenses for these industries. These R&D expenses are driven by costly protocols and the incidences of rework and retesting based on the inherent inaccuracies.
The above-mentioned industries are exploring novel models to drive productivity up and cost down, while more importantly moving away from cruel and animal-based testing. Specific attention is required for new market entrants to provide a smooth method change-over and continuity of R&D operations. Currently, wider adoption is hindered by the limitations of traditional technologies used for the culturing and testing of tissue explants and cell cultures. These limitations include low complexity of 2D and even 3D models, too simplistic recreations of the cells/tissue microenvironment, laborious manual testing operations, and low throughput.
The global need to find accurate and reliable alternatives to animal models and a competitive CRO market are pushing the development of realistic ex vivo and in vitro technologies. This will help reduce failure rates as well as the occurrence of too many false positives and false negatives, hence preventing expensive rework and errors in the end-to-end product development process. This is a particularly pressing problem for those human tissues that have a barrier function against external agents such as skin, oral and nasal mucosa, respiratory epithelia, and the like. Additionally, significant process improvements are needed to optimise and accelerate the development of novel consumer products and therapeutics for human use.
The solution
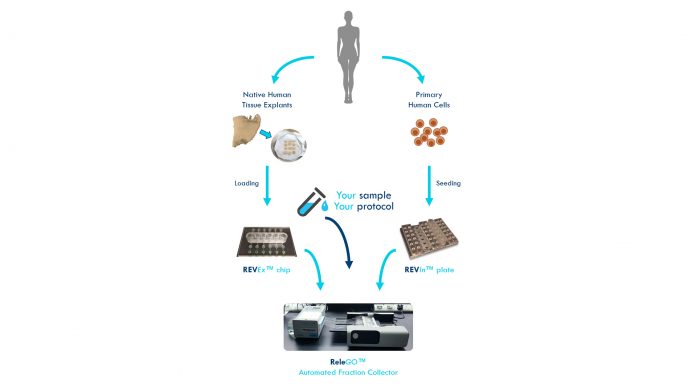
REVIVO BioSystems has developed an organ-on-a-chip platform for the realistic and time/cost-optimised assessment of ingredients, formulations, and finished products. Such platform combines the principles of miniaturisation and microfluidics with those of biological transport phenomena and tissue engineering. This integration provides the most realistic human barrier tissue models achievable in lab conditions. REVIVO BioSystems is thus offering a highly representative, automated testing platform that is accurate, efficient, and cost effective. The company is passionate about making significant contributions to a cruelty-free research environment, from reducing and even eliminating animal tests, all the way down to removing animal-derived components in its own tissue cultivation processes.
REVIVO BioSystems’ complete solutions include:
· Microfluidic devices designed for use with tissue explants (REVEx™ chip) and/or for culturing organotypic epithelial tissue models (REVIn™ plate) under dynamic conditions;
· An automated testing instrument (ReleGO™) including the functions of fraction collection, temperature control, and enabled for electrode/sensor integrations, in which the microfluidic devices are loaded; and
· 4D tissue models (explants or full-thickness cell cultures).
Biomimicry and miniaturisation are key features offered by the REVEx chip and the REVIn plate, while ReleGO enables automation.
REVIVO BioSystems offers a more efficient and ethical way to reach the market faster while lowering its customers’ R&D expenses (a five-fold reduction in biomaterial consumption, and a 10-fold reduction in specialised manpower), but also highly realistic 4D tissue models that accelerate the product testing and validation processes.
REVIVO BioSystems’ testing platform
Tissue explants (sourced directly by REVIVO BioSystems or obtained from authorised suppliers) and tissue engineered models are loaded and grown, respectively, into the microfluidic chips. A continuous flow of nutrients and reagents through the microfluidic channels mimics the function of the blood flow, thus providing 4D tissue models. This creates a unique micro-environment that establishes natural tissue properties. Continuous flow further enables the long-term maintenance of the human tissues and hence the potential to study the biological response over a few weeks (instead of a few days). The flow rate can be fine-tuned and the collection of the outflow is performed automatically at custom-defined time intervals in 96-well collection plates using ReleGO. This enables the end user to have a kinetic analysis with a 10-fold reduction of man-hours otherwise spent in tedious manual operations.
Both topical and systemic product dosages can be studied, with a realistic physiological response. Direct access to the tissues’ external surface enables the topical applications of not only solids, semisolids, and liquids, but also gases and aerosols. Drugs or test compounds can also be delivered via the microfluidic channels, thus mimicking a systemic exposure. Measurement of transepidermal electrical resistance is also enabled, a parameter frequently used to assess the barrier integrity of epithelial tissues.
REVSkin™ and 4D epithelial cultures
REVSkin is a first of its kind, proprietary, full-thickness skin-on-chip equivalent reconstructed under dynamic culture conditions (4D skin model). The continuous flow of fresh nutrients and the draining away of metabolic wastes enabled by REVIVO BioSystems platform helps mimick the function of blood vessels, unlike traditional inserts-based static 3D cultures. As a result, REVSkin shows natural morphology, enhanced maturation, and improved barrier function.3
It includes both epidermis and underlying dermis and is biofabricated using:
• Primary human cells (thus offering the potential for customisation and even personalisation);
• Non-contracting, fibrin-based dermal matrix (collagen present in REVSkin is produced by the human cells seeded in the model, making it an ideal tool for anti-ageing studies); and
• Culture media free of animal serum (thus avoiding exogenous and animal-derived products such as rat-tail or bovine collagen).
Adoption of REVSkin is driven by the ban on animal testing for cosmetic product development (2013 EU ban) and for the assessment of chemicals (USA’s Environmental Protection Agency 2019 work plan) on a global scale.
REVSkin is the initial instalment of a series of 4D full-thickness epithelial equivalents that REVIVO Biosystems is developing for modelling external biological barriers for personal care, immunotherapy, and viral infection studies. Epithelial tissues serve as protective barriers against chemical, physical, thermal, and microbial insults. Hence, the evaluation of safety, absorption, and efficacy of personal care, chemical, and pharmaceutical products that come into contact or need to permeate these biological barriers is of paramount importance prior to their use in humans.
Case study one: explants-on-chip
The REVEx chip is a six-chamber microfluidic device where six tissue explants can be loaded, cultured, and used for testing. Only an 8mm disc of specimen is required per replicate, i.e. four to five times smaller specimen than in traditional diffusion cells. It can also be used with commercial reconstructed tissue equivalents, synthetic membranes, and any biological barrier simulant.
Two REVEx chips can be loaded onto the ReleGO instrument to provide the first programmable, automated, and hassle-free solution for ex vivo testing needs. A high level of automation of the testing procedures is achievable while enabling realistic in vivo-like permeation, safety, and efficacy studies. For example, it allows automated permeation testing in a flow-through design at a fraction of the material cost of traditional permeation systems. It yields higher precision, allows the quantification of lower permeation rates, and reduces the problem of unstirred water layer.2
Case study two: 4D epithelial cultures
The REVIn system is designed to culture full-thickness epithelial models at the air-liquid interface under dynamic conditions. The biofabrication of full-thickness tissue-engineered equivalents primarily entails the culture of cells in and over a three-dimensional matrix. The microchannels below the culture chambers allows for the flow of nutrients that mimics the blood flow, while the microchannels above allow for the flow of humidity-controlled air and/or media. The active flow of nutrients and air better mimics the native microenvironment compared to static cultures.
This is similar to native skin, for example, wherein the outer surface is exposed to air while the underlying cells derive nutrition from blood vessels. When used for the fabrication of tissue engineered full thickness skin models, the REVIn system ensure the formation of REVSkin, a 4D model that better mimics native human skin.3 The flows of media and air induce mechanotransduction signalling leading to the superior maturation of basement membrane proteins. This feature is crucial as these proteins are involved in anchoring the epidermis to the underlying dermis. The enhanced maturation and barrier function of REVSkin combined with the use of a non-contracting fibrin-based dermal matrix has been shown to improve the reliability of skin permeation experiments and other toxicity tests that depend on skin exposure.
Challenges and future directions
REVIVO BioSystems is a spin-off from the Singapore Agency for Science Technology and Research (A*STAR) and is based in the vibrant biomedical ecosystem in Biopolis (Singapore). Its founding team of engineers, biologists, and clinician scientists has extensive experience in biomedical engineering, cell cultures, microfluidics, and tissue engineering. The team came together with a passion to become the Asian and global leading provider of enabling technologies and services for the ex vivo and in vitro testing of chemicals, ingredients, cosmetic formulations, and therapeutics.
The invention and development of the organ-on-chip technology occurred in a collaborative effort between the Singapore Institute of Manufacturing Technology and the Institute of Medical Biology. In 2019, an Innovation Grant from the Singapore MIT Alliance for Research and Technology Centre was granted to further mature the technology. In June 2020, the skin testing service platform was commercially launched with leading corporate customers. The company is now scaling operations and building a steady pipeline of customers, while deepening the portfolio with existing ones to enable companies that require skin testing services to transition from traditional methods to our platform.
As of today, REVIVO BioSystems is raising capital to primarily accelerate its capacity to scale-up for contract testing services. It is also finalising the scale-up of product manufacturing to be in a position where it can offer the direct sale of testing equipment and supplies.
Moving forward, REVIVO BioSystems is on a mission to eliminate cruelty to animals, increase the efficiency of product testing, and enable personalisation in the chemical, personal care, and pharmaceutical industries. The aim is to transform the in vitro research market and make it scalable, personalised, and ethical.
References
1. Meigs, L. et al. ‘Animal testing and its alternatives – the most important omics is economics’, ALTEX 2018;35(3):275-305. DOI: 10.14573/altex.1807041
2. Alberti, M. et al. ‘Multi-chamber microfluidic platform for high-precision skin permeation testing’, Lab Chip 2017;17(9):1625-1634. DOI: 10.1039/c6lc01574c.
3. Sriram, G. et al. ‘Full-thickness human skin-on-chip with enhanced epidermal morphogenesis and barrier function’, Materials Today 2018;21(4):326-340. DOI: 10.1016/j.mattod.2017.11.002
Dr Massimo Alberti
CEO/Co-Founder
Dr Bert Grobben
CBO/Co-Founder
Dr Sriram Gopu
Scientific Advisor/Co-Founder
REVIVO BioSystems Pte Ltd
info@revivobio.com
www.revivobio.com
www.linkedin.com/company/revivo-biosystems
Please note, this article will also appear in the fourth edition of our new quarterly publication.