Professor Dietmar W Hutmacher, Director of the ARC Centre in Additive Biomanufacturing at the Queensland University of Technology, discusses how scaffold-guided tissue engineering can potentially improve outcomes of breast reconstruction following mastectomy.
Breast reconstruction following mastectomy has long been approached in two main ways: implantation; and autologous reconstruction. Both of these methods present different challenges to the patient and surgeon. Herein we present the role scaffold-guided tissue engineering can play in solving this complex surgical problem.
Defects following oncologic resection of breast cancer have a significant impact on a woman’s quality of life through changes in body image, sexual function, and psychological state.1 It is for this reason that discussion and access to reconstructive options are considered a necessary component to the interdisciplinary management of breast cancer.2
Despite this obvious need, patient geography, access to plastic surgery, surgical and patient preference, and finances limit uptake of breast reconstruction.3, 4 In this article, we will discuss how scaffold-guided tissue engineering can potentially improve outcomes of breast reconstruction following mastectomy.
Scaffold-guided tissue engineering
The fundamental concept underlying scaffold-guided tissue engineering is to combine a scaffold or matrix, with living cells, and/or biologically active molecules to form a ‘tissue engineering construct (TEC)’ to promote the repair and/or regeneration of tissues. The scaffold is expected to perform various functions, including the support of cell colonisation, migration, growth, and differentiation. In addition, the design of these scaffolds needs to consider physio-chemical properties, morphology, and degradation kinetics. The external size and shape of the construct are also of importance, particularly if the construct is customised for an individual patient.
Scaffolds must provide sufficient initial mechanical strength and stiffness to withstand the biomechanical forces at the defect side. Yet, scaffolds may not necessarily be required to provide complete mechanical equivalence to healthy tissue, but stiffness and strength should be sufficient to at least support and transmit forces to the host tissue site in the context.
Cell and tissue remodelling are important for achieving stable biomechanical conditions and vascularisation at the host site. Hence, the scaffold should maintain sufficient structural integrity during the in vitro and/or in vivo growth and remodelling process. The degree of remodelling depends on the tissue itself (e.g. skin four to eight weeks, bone six to 12 months), and its host’s anatomy and physiology. In addition to these essentials of mechanics and geometry, a suitable scaffold will possess surface properties which are optimised for the attachment and migration of cell types of interest (depending on the targeted tissue). The external size and shape of the construct must also be considered; especially if the construct is customised for an individual patient (Hutmacher, 2000).
Degradation rate
The degradation rate of scaffolds is one of most important considerations and it is highly desirable to ensure that not only the degradation rate but also the tissue remodelling and maturation matches with new tissue regeneration at the defect site. In the early days of tissue engineering it was stated that the scaffold should vanish away as the tissue grows. Yet, this process is different for each tissue from a time point of view, while tissue growth does not equal tissue maturation and then remodelling. Hence, many scaffold-based strategies studied in the literature did fail, as the biomaterial degradation was more rapid than the tissue regeneration and/or remodelling.
We proposed a paradigm shift in the field by designing scaffolds that only start the degradation after the regenerated tissue inside the scaffolds has remodelled at least once or twice in the natural tissue remodelling cycle. This concept is described in detail in Fig. 1 for scaffold guided bone regeneration.
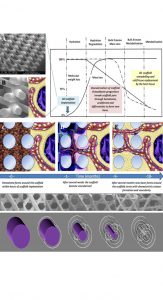
Our group pioneered the use of medical grade polycaprolactone (mPCL) as a scaffold for soft tissue regeneration combined with autologous fat graft (see Fig. 2).5 mPCL has been successfully used to develop a large number of FDA-approved medical devices. This polymer has a slow degradation rate (approximately two to four years for complete degradation in vivo depending on the molecular weight and implant volume) and high plasticity with excellent 3D melt printability and favourable mechanical properties.
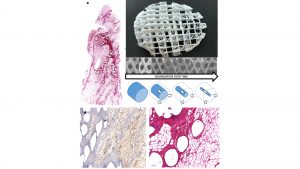
Over the last decade, several studies have demonstrated the biocompatibility and tissue regeneration support of MEW mPCL scaffolds.6 Because of its many merits, PCL has been widely used to promote bone tissue regeneration.7,8,9 The use of medical grade polymer in the research phase facilitates the translation into a regulatory approved product and reduces the risk of residual toxic components in the finished product.
Autologous fat grafting
Autologous fat grafting has been used in medicine for over 100 years. However, today it still has a resorption rate of up to 25-70%.10 The scaffold functions as a mechanical support to prevent overload of the newly-forming tissue inside the scaffold architecture, as well as in the later stage of regeneration as a guide for angiogenesis (Fig. 2 a, d, e.) By performing these functions, the scaffold addresses the key mechanisms that underly what is defined by plastic surgeons as adipose tissue graft take.11,12
To date, we have successfully applied this method of reconstruction in both small and large animal studies. Chhaya et al. (2015) demonstrated sustained large volume adipose tissue regeneration in a murine model with a PCL scaffold.13 This model’s results were successfully scaled up in a pig model.14 Chhaya et al. (2016) further expanded on the regenerative potential of the scaffold by applying the ‘body as a bioreactor’ principle – whereby delayed lipo-aspirate was injected into pre-implanted empty scaffolds, demonstrating improved graft survival due to the pre-vascularisation allowed by delay between procedures. Here, they successfully implanted 100ml mPCL scaffolds in a minipig model. We are currently conducting a larger volume (>300ml) scaffold experiment with a minipig animal model which we hope will further bridge the gap to clinical application with volumes that reflect those in mastectomy defects.
In addition to the regenerative properties of the scaffold, mPCL can also be a potential drug delivery device. In vitro studies have shown sustained release of antibiotics from mPCL scaffolds with a microporous architecture for days following loading.15 This development further expands that potential scope of our scaffolds to secondary reconstruction following implant-related complications given that bacterial colonisation has been implicated in implant-based complications including capsular contracture and the emerging breast implant-associated anaplastic large cell lymphoma (BIA-ALCL). Further studies are required to substantiate early in vitro promise in this field.
This approach can also be applied to reconstruction of other complex soft tissue defects. Given the customisable nature of the design process, defects such as congenital pectus excavatum can also be reconstructed to provide patients with major social and psychological difficulties a viable camouflage option.
Our proposed SGBTE could potentially offer patients the optimal reconstructive option, given that the material is biodegradable and eventually leaves that patient with only their own tissue, minimal donor site morbidity, involves a routine operative procedure, and has been shown to be effective for reconstructing large volume defects.
References
- Flitcroft, K., M. Brennan, and A. Spillane, ‘Decisional regret and choice of breast reconstruction following mastectomy for breast cancer: A systematic review’. Psychooncology, 2018. 27(4): p. 1110-1120
- Brennan, M.E. and A.J. Spillane, ‘Uptake and predictors of post-mastectomy reconstruction in women with breast malignancy – systematic review’. Eur J Surg Oncol, 2013. 39(6): p. 527-41
- Nahabedian, M.Y., ‘Acellular dermal matrices in primary breast reconstruction: principles, concepts, and indications’. Plast Reconstr Surg, 2012. 130(5 Suppl 2): p. 44S-53S
- Weichman, K.E., et al., ‘The use of acellular dermal matrix in immediate two-stage tissue expander breast reconstruction’. Plast Reconstr Surg, 2012. 129(5): p. 1049-58
- Melchels, F., et al., ‘CAD/CAM-assisted breast reconstruction’. Biofabrication, 2011. 3(3): p. 034114
- Dang, H.P., et al., ‘Porous 3D Printed Scaffolds For Guided Bone Regeneration In a Rat Calvarial Defect Model’. Applied Materials Today, 2020. 20: p. 100706
- Eskandarinia, A., et al., ‘A Novel Bilayer Wound Dressing Composed of a Dense Polyurethane/Propolis Membrane and a Biodegradable Polycaprolactone/Gelatin Nanofibrous Scaffold’. Scientific Reports, 2020. 10(1): p. 1-15
- Ehterami, A., et al., ‘In vitro and in vivo study of PCL/COLL wound dressing loaded with insulin-chitosan nanoparticles on cutaneous wound healing in rats model’. International journal of biological macromolecules, 2018. 117: p. 601-609
- Chong, E.J., et al., ‘Evaluation of electrospun PCL/gelatin nanofibrous scaffold for wound healing and layered dermal reconstitution’. Acta biomaterialia, 2007. 3(3): p. 321-330
- Bellini, E., M.P. Grieco, and E. Raposio, ‘The science behind autologous fat grafting’. Ann Med Surg (Lond), 2017. 24: p. 65-73
- Khouri, R.K., Jr., et al., ‘Diffusion and perfusion: the keys to fat grafting’. Plast Reconstr Surg Glob Open, 2014. 2(9): p. e220
- Eto, H., et al., ‘The fate of adipocytes after nonvascularized fat grafting: evidence of early death and replacement of adipocytes’. Plast Reconstr Surg, 2012. 129(5): p. 1081-92
- Chhaya, M.P., et al., ‘Sustained regeneration of high-volume adipose tissue for breast reconstruction using computer aided design and biomanufacturing’. Biomaterials, 2015. 52: p. 551-60
- Chhaya, M.P., et al., ‘Transformation of Breast Reconstruction via Additive Biomanufacturing’. Sci Rep, 2016. 6: p. 28030
- Visscher, L.E., et al., ‘3D printed Polycaprolactone scaffolds with dual macro-microporosity for applications in local delivery of antibiotics’. Mater Sci Eng C Mater Biol Appl, 2018. 87: p. 78-89
Co-Authors
Dr Michael Wagels
Clinical Director, Biofabrication for Surgery
Herston Biofabrication Institute (HBI)
Royal Brisbane and Women’s Hospital
hbi@health.qld.gov.au
Owen A Ung FRACS FAICD
Professor of Surgery
University of Queensland
Director, MNHHS Comprehensive Breast
Cancer Service
Breast and Endocrine Surgeon
Royal Brisbane and Women’s Hospital
o.ung@uq.edu.au
Author
Distinguished Professor Dietmar W Hutmacher
PhD (NUS), MBA (Henley), FAHMS
Director, ARC Centre in Additive Biomanufacturing
QUT Chair in Regenerative Medicine,
Institute of Health and Biomedical Innovation
Queensland University of Technology
+61 7 3138 6077
dietmar.hutmacher@qut.edu.au
Tweet @ARC_ABM
www.qut.edu.au/institute-of-health-and-biomedical-innovation
Please note, this article will also appear in the fourth edition of our new quarterly publication.