A team of researchers has developed aluminium-ion batteries with an organic redox polymer as a positive electrode material.
When produced with this material, aluminium-ion batteries stored an unprecedented 167 milliampere hours per gram. This outperforms batteries using graphite as an electrode material.
The team, led by researchers from the University of Ulm and the University of Freiburg, developed an anode that consisted of an organic redox polymer based on phenothiazine.
A paper detailing the research, ‘On a high-capacity aluminium battery with a two-electron phenothiazine redox polymer as a positive electrode,’ was published in Energy & Environmental Science.
Aluminium: A promising alternative battery material
Due to it being one of the most common elements in Earth’s crust, aluminium is seen as a promising alternative to conventional batteries that use scarce and difficult-to-recycle raw materials such as lithium.
In comparison, aluminium-ion batteries are easier to recycle, safer, and less expensive than batteries that use other materials.
Within the organic redox polymer, aluminium surpasses the capacity of graphite, which is currently the most commonly used electrode material.
Using complex ions in the electrode material
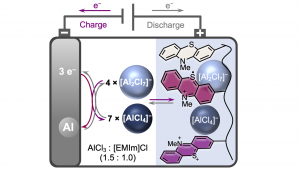
The electrode material is oxidised during the charging of the battery, where it takes up complex aluminate anions.
The organic redox polymer, poly(3-vinyl-N-methylphenothiazine), managed to insert two [AlCl4]− anions reversibly during charging. The researchers used the ionic liquid chloride as an electrolyte with added aluminium chloride.
“The study of aluminium-ion batteries is an exciting field of research with great potential for future energy storage systems,” said Gauthier Studer, who led the research.
Studer continued: “Our focus lies on developing new organic redox-active materials that exhibit high performance and reversible properties.
“By studying the redox properties of poly(3-vinyl-N-methylphenothiazine) in chloroaluminate-based ionic liquid, we have made a significant breakthrough by demonstrating for the first time a reversible two-electron redox process for a phenothiazine-based electrode material.”
Aluminium-ion batteries retain an outstanding storage capacity
The redox polymer of the aluminium-ion batteries deposits the anions at potentials of 0.81 and 1.65 volts and provides specific capacities of up to 167 mAh/g.
In contrast, the discharge capacity of graphite as an electrode material in batteries is 120 mAh/g.
After 5,000 charge cycles, the battery presented by the research team still retains 88% of its capacity at 10 C, for example, at a charge and discharge rate of six minutes. At a lower C rate (a longer charge and discharge time), the aluminium-ion battery returns unchanged to its original capacities.
Birgit Esser, another of the study’s lead researchers, concluded: “With its high discharge voltage and specific capacity, as well as its excellent capacity retention at fast C rates, the electrode material represents a major advance in the development of rechargeable aluminium-ion batteries.
“Our concept provides the battery industry with advanced and affordable energy storage solutions.”