Nathan Stasko, Chief Executive Officer of Vast Therapeutics, states that patients deserve new treatment options like ALX1 in the fight against antibiotic-resistant bacteria.
Chronic lung diseases lead to a harmful cycle of inflammation and infection. This can result in trouble breathing, frequent hospital stays, and, sadly, even death. Antibiotic-resistant bacteria have evolved defences against some of the medical field’s best drugs and have set up base camp in the lungs of patients with cystic fibrosis (CF), non-CF bronchiectasis (NCFB), and chronic obstructive pulmonary disease (COPD).
At Vast Therapeutics (‘Vast’), our mission is to develop innovative therapies that address the root causes of these debilitating conditions, offering hope for a brighter future. Vast is led by a strong team of experienced scientists and clinicians who are passionate about developing life-changing treatments. Our team brings over 120 years of drug development experience, including leadership roles in 20 FDA-approved new drug applications (NDAs). With our proprietary nitric oxide technology and a commitment to patient-centric care, we believe we are well-positioned to address the significant unmet medical need in respiratory medicine and the global problem of antimicrobial resistance.
Vast’s goal: To eradicate Pseudomonas aeruginosa
While there are several respiratory diseases with significant unmet needs, our initial efforts are concentrated on population segments whose lungs are colonised with the multi-drug-resistant and potentially life-threatening bacteria Pseudomonas aeruginosa (Pseudomonas). It is now well understood that, when CF and NCFB patients are colonised with Pseudomonas, it leads to higher rates of hospitalisation and mortality.1 Cough, excessive sputum, and difficulty breathing become part of everyday life, leading to exacerbations, chronic airflow obstruction, and progressive loss of lung function. The heavy use of chronic antibiotics to treat these diseases has led to toxicities observed with long-term use and antibiotic resistance (further complicating effective treatment).2
The need for new treatments that do not develop antimicrobial resistance creates an opportunity to improve and extend life for thousands of people living with Pseudomonas. Outside of CF, there are no FDA-approved treatments specifically indicated for Pseudomonas airway infections. Within CF, Pseudomonas remains a significant challenge to adult patients over the age of 25 and for those ~10% of individuals with genetics that are ineligible for corrective therapy.
ALX1 drug candidate
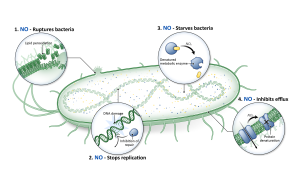
To reach these patients, we have developed a drug candidate designed to deliver broad-spectrum antimicrobial activity with minimal toxicity and no evidence of emerging drug resistance in laboratory studies to date. It uses a natural molecule produced by the body called nitric oxide (NO).
Nitric oxide is one of the most researched molecules in human physiology. As a fundamental part of host immune response, cells of the immune system naturally generate nitric oxide to kill bacteria, fungi, and viruses. Due to the multiple ways nitric oxide kills bacteria, they do not develop resistance like they do to traditional antibiotics (see Figure above).
While the powerful benefits of nitric oxide have long been recognised, there is a scarcity of nitric oxide-based medicines on the market due to the challenges associated with controlling the release and delivery of nitric oxide because it is a gas in its natural state. Early data indicates that our nitric oxide chemistry platform pioneered by Dr Mark Schoenfisch, jointly appointed Professor in the School of Medicine and School of Pharmacy at UNC-Chapel Hill, overcome these limitations.
ALX1 utilises nitric oxide, stabilised in liquid form, to kill Pseudomonas and other antibiotic-resistant bacteria. A fine mist is delivered daily to the lungs via a hand-held, portable inhalation device similar to an inhaler. Vast has created an easy-to-use solution to a complex problem.
There is a timely need for effective new treatments to eradicate Pseudomonas and other drug-resistant bacteria in an effort to combat the global public health threat of antibiotic resistance. Based on superior efficacy compared to tobramycin in animal models3 we believe our lead product candidate, known as ALX1, can potentially eliminate Pseudomonas in the lungs of patients.
ALX1 is now ready for Phase 1 clinical trials. Vast has the ability to manufacture the drug in high purity and an approval from the Food and Drug Administration (FDA) to begin safety trials in humans. Completion of Phase 1 in healthy volunteers then enables proof-of-concept testing in patients with Cystic Fibrosis and other non-CF diseases in the broader population. If successful, ALX1 may eventually become part of the standard-of-care treatment regimen in many airway diseases.
We’ve heard the call, and encourage others to feel the urgency of time for these patients.
References
- Reynolds D, Kollef M. ‘The Epidemiology and Pathogenesis and Treatment of Pseudomonas aeruginosa Infections: An Update,’ Drugs 2021, Volume 81, 2117–2131.
- Horne M, Woolley I, Lau JSY. ‘The Use of Long-term Antibiotics for Suppression of Bacterial Infections,’ Clinical Infectious Diseases, 2024, Volume 79, 848–854
- Schoenfisch M, Hollenbach S, Simons JK, Stasko N. ‘Efficacy of Inhaled ALX1 in a Model of Chronic Pseudomonas Aeruginosa Infection,’ Respiratory Drug Delivery 2024. Volume 1, 2024: 508-511.
Please note, this article will also appear in the 20th edition of our quarterly publication.