Dr Björn M Burmann, from the University of Gothenburg, outlines the cellular clean-up process from a molecular level.
In the real world, waste is normally disposed of and a similar process also needs to be achieved within the biological context of the cell to get rid of broken and possible harmful proteins. Where this task would be achieved in the outside world by a cleaning team, this job is carried out in cells by proteins known as proteases. These proteases work in close relationship to another class of proteins, molecular chaperones, that might be able to rescue and salvage some misfolded proteins to regain their functionality. These processes are of great importance for the cellular fitness, as you can imagine what happens when a maintenance crew is on strike in a big city. A non-functional system in the cells can also directly lead to a non-functional cell through the accumulation of broken proteins, a problem found at the onset of a wide range of severe diseases.
Maintaining cellular fitness
Researchers from the Department of Chemistry and Molecular Biology and the Wallenberg Centre for Molecular and Translational Medicine of the University of Gothenburg, Sweden, used Escherichia coli – a bacterium found in the human gut microbiota that has adapted to survive under a wide variety of different environmental stress conditions – as a model system. Within this bacterial species, one of the main components of the bacterial stress response to heat is an enzyme called DegP – a protease which can shred unstable proteins to prevent them from accumulating in the cell envelope of bacteria. DegP is inactive at low temperatures and only becomes active at elevated temperatures, however, the molecular mechanism underlying its activation remained unknown so far.
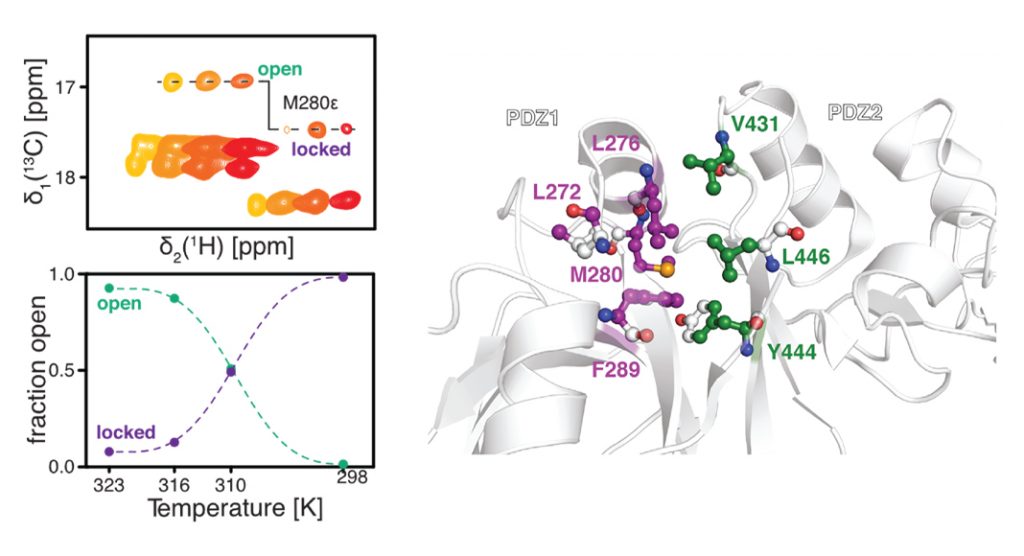
Dr Burmann explained: “DegP in the inactive resting state forms a stable hexamer – a protein formed by six subunits – which splits into trimers (of three subunits) as the temperature increases. This splitting unleashes the destructive activity of the protease, allowing it to efficiently remove unwanted proteins. By using nuclear magnetic resonance (NMR) spectroscopy, the team could show in detail how this mechanism is governed by two regulatory domains of DegP. At low temperatures, these domains are stabilised by a molecular lock forming the hexamer. However, at high temperatures, the individual domains become more dynamic and consequently separate into active trimers (Fig. 1).
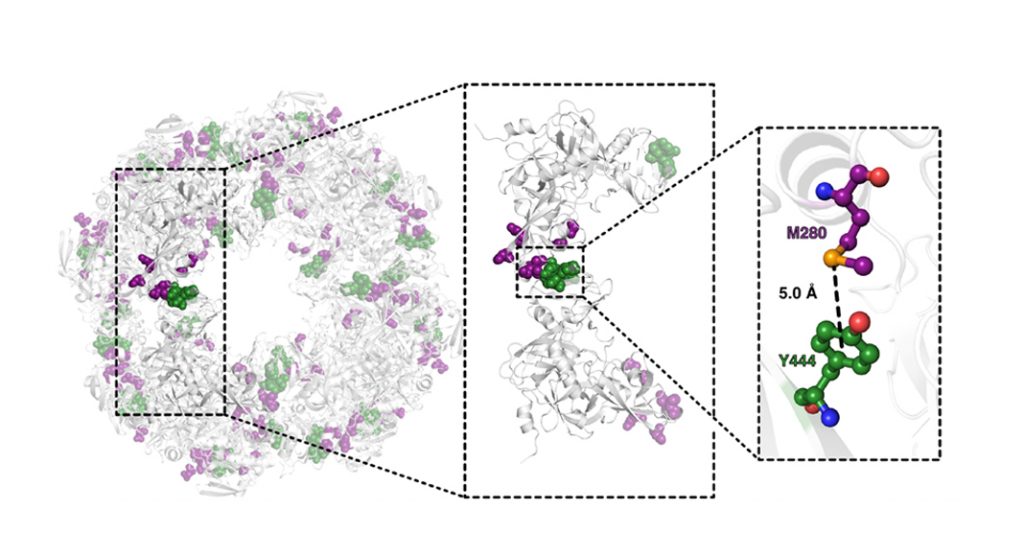
“Remarkably, DegP exploits a similar interdomain lock to stabilise the proteolytic cage for efficiently shred misfolded proteins that could be structurally described already before by X-ray crystallography (Fig. 2).
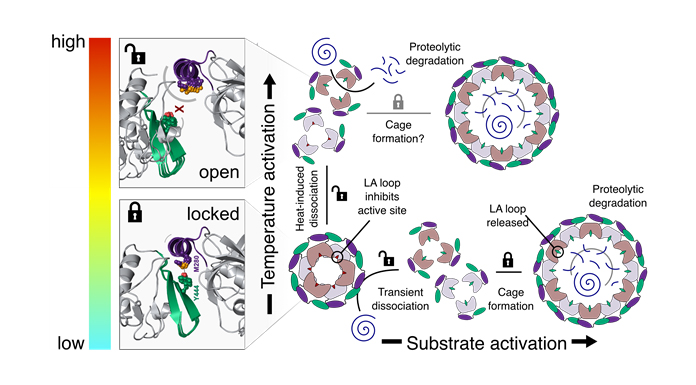
“Overall, the newly characterised built-in activation mechanism permits DegP self-regulation (Fig. 3), which can be hopefully exploited for antimicrobial drug research. Furthermore, this molecular activation mechanism seems to be evolutionarily conserved for this class of proteins, providing also fresh new insight for related human proteases involved in different types of cancer and neurodegeneration.”
Facts
Proteases are enzymes, biological catalysts, that facilitate proteolysis – the break-down of proteins into smaller peptides or even individual amino acids.
The research was carried out using the outstanding NMR spectroscopy infrastructure at the Swedish NMR Centre, hosted by the University of Gothenburg.
This research was made possible by generous funding by the Knut och Alice Wallenberg Foundation (BMB), the Vetenkapsrådet (BMB,) as well as an EMBO Long-Term Fellowship to co-author Johannes Thoma.
References
1 Šulskis, D., Thoma, J. & Burmann, B. M. Structural basis of DegP protease temperature-dependent activation. Sci. Adv. 7 (2021).
2 Krojer, T. et al. Structural basis for the regulated protease and chaperone function of DegP. Nature 453, 885-890 (2008).
Please note, this article will also appear in the ninth edition of our quarterly publication.








